Payer Reaction To FDA Safety Warnings — What Pharma Manufacturers Need To Know

By Jeremy Schafer, Frank Cathers, And Sejal Jonas, Precision For Value
Safety warnings on previously unknown adverse events can significantly impact a medication. In 2007, telithromycin (Ketek) was a market leading oral antibiotic.1 However, a report in the Annals of Internal Medicine detailing three cases of severe liver failure associated with telithromycin led to new FDA-imposed restrictions. Sales of telithromcyin plunged soon afterwards.1-2 A similar fate befell rosiglitazone (Avandia) when a meta-analysis led to updated FDA label warnings regarding cardiovascular risk.3
Safety warnings do not necessarily mean the commercial end of a product. Natalizumab’s (Tysabri) progressive multifocal leukoencephalopathy (PML) issue led to a temporary market withdrawal and rerelease with new safety monitoring. Natalizumab is now an important agent for the treatment of multiple sclerosis.4
Regardless, new safety warnings create a complex issue for payers and manufacturers. Payers must attempt to protect patient safety on a population level but also avoid blocking access of the drug to those benefiting from therapy. Little is known about how payers react to safety events in the short term. Insights into initial payer reactions to new safety warnings may provide information on how an issue is managed in the short term and what data a payer would value from the manufacturer.
On October 22, 2015, the FDA released a statement describing updated liver safety warnings for Viekira Pak. Data indicated that Viekira Pak was associated with 26 cases of medication-associated liver injury. Stakes were high in the multibillion dollar hepatitis C (HCV) market, with the fate of one of only two major competing products now unknown. Precision For Value sought to understand the payer reaction by studying payer viewpoints related to a new safety signal in the HCV space.
Survey Methodology
Precision for Value leveraged its proprietary survey tool, Pivot, to conduct research among payers in response to the new warning for Viekira Pak. Pivot is a smartphone application that allows for responses entirely on a respondent’s phone. A multiple-choice, 13-question survey was developed and launched on October 23, 2015 at 6 p.m. EST, less than 24 hours after the release of the safety warning. The survey was closed on October 26, 2015, with 39 respondents representing 200 million commercial lives having responded.
Results And Interpretation
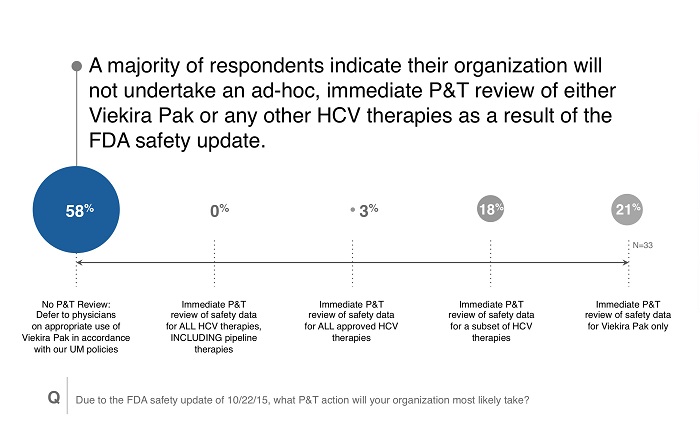
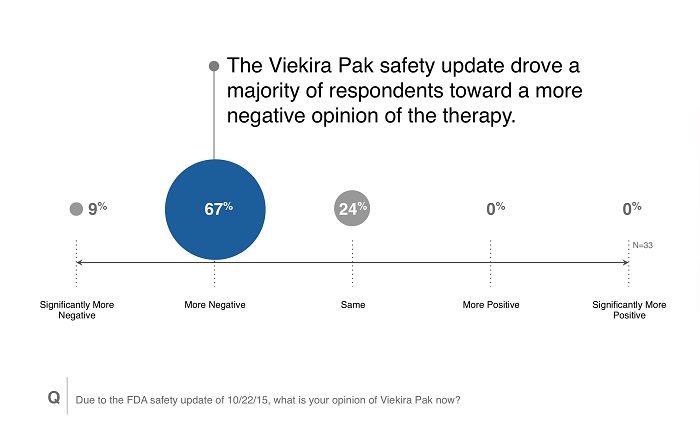
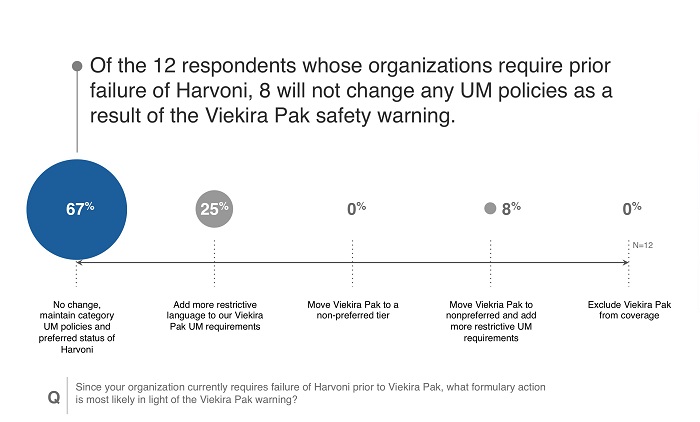
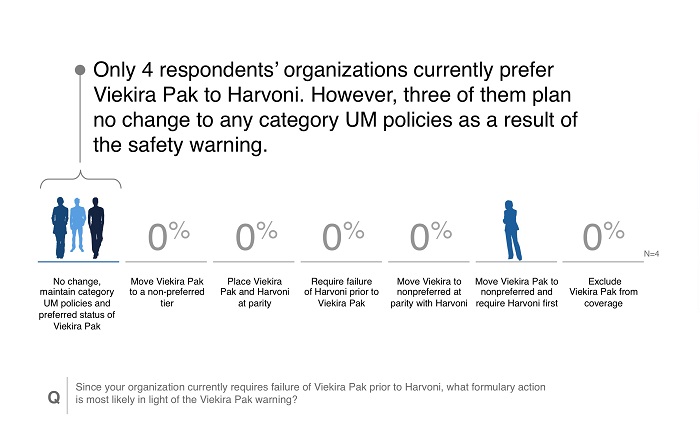
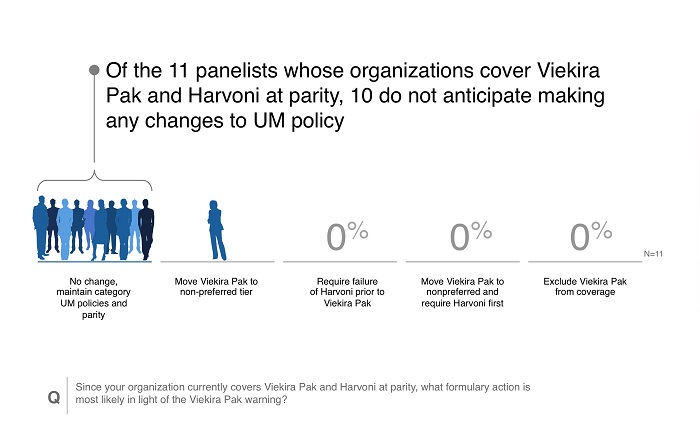
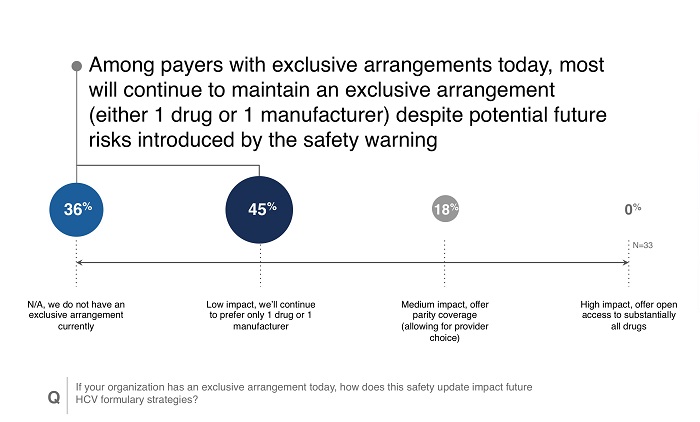
Considerations
Reaction to new safety warnings can be swift, and healthcare stakeholders often err on the side of caution while further data is researched. A rapid reaction can have the benefit of protecting patients but may also restrict access. The responses from the survey indicate that payers were taking a cautious approach to the new safety signals of Viekira Pak, with few making moves to change controls. The reasons for these findings are multifactorial and may include the following:
- Over half of respondents were at payers that already preferred Harvoni to Viekira Pak, meaning Viekira Pak utilization was likely limited.
- The safety issues arose predominantly in patients with Child-Pugh B liver disease, an “off label” use for Viekira Pak. Existing payer criteria may already restrict access to these patients.
- Payers may be unsure of the causality of Viekira Pak, since advanced HCV patients already are at high risk of liver failure.
Regardless of survey reaction, the new safety warning gave payers a more negative impression of Viekira Pak than they had prior to the warning. While formulary status for Viekira Pak may not be altered, the new viewpoint could make Viekira Pak more vulnerable to competitors in the HCV space, particularly with the recent approval of Zepatier. Payers did indicate overwhelmingly that they would consider or keep single preferred product arrangements in HCV.
New safety warnings can go beyond the implicated product and dampen an entire class of medications. The analysis did reveal that some payers would review the safety of all HCV products. In order to address payer concerns and reduce risk of being swept up in a market reaction, manufacturers in similar scenarios can provide value in several ways.
- Share as much data as possible related to the new safety issue in question with the payer, including both real-world and clinical trial experience.
- Leverage health outcomes research when possible to assist a payer’s investigation within its own population.
- Discuss safety monitoring programs with the payer for proactive identification of safety issues.
- Engage medical affairs teams to educate payers on the safety warning, and provide insights into appropriate policy updates.
All manufacturers that have products within an impacted category should be prepared to speak to how the safety implications may or may not apply to their product. Payers need to understand the implications of the issue and how to manage their members accordingly. The manufacturer is best positioned to provide the information that is meaningful and relevant.
Conclusion
New safety warnings can be significant market events, and data is often limited in the short term. Precision For Value’s analysis underscores the importance of examining payer reaction to new safety warnings. The findings indicate that payer reaction to the Viekira Pak warning was cautious but evolving. It’s critical for manufacturers to be proactive and transparent in providing payers with accurate, timely, and detailed information to prevent misinformed coverage decisions.
References
- Gleason PP, Walters C, Heaton AH, Schafer JA. Telithromycin: the perils of hasty adoption and persistence of off-label prescribing. J Manag Care Pharm. 2007;13(5):420-425.
- Clay KD, Hanson JS, Pope SD, Rissmiller RW, Purdum PP, Banks PM. Brief communication: severe hepatotoxicity of telithromycin: three case reports and literature review. Ann Intern Med. 2006;144:415-20.
- Starner CI, Schafer JA, Heaton AH, Gleason PP. Rosiglitazone and pioglitazone utilization from January 2007 through May 2008 associated with five risk-warning events. J Manag Care Pharm 2008;14(6):523-531.
- FDA. FDA Approves Resumed Marketing of Tysabri Under a Special Distribution Program. June 5, 2006. Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2006/ucm108662.htm
- FDA. Hepatitis C Treatments Viekira Pak and Technivie: Drug Safety Communication - Risk of Serious Liver Injury. October 22, 2015. Available at: http://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm468757.htm
About The Authors
Jeremy Schafer is VP of specialty strategy at Precision for Value. He has experience in specialty pharmacy programs and networks, P&T Committee processes and review, prior authorization programs, fee schedule management, medical and pharmacy benefit integration, Medicare Part D, health outcomes research, contracting (rebate and specialty pharmacy), health plan and PBM strategy, and payer data analysis. He received his MBA from Bethel University and his Doctor of Pharmacy degree from the University of Minnesota in Minneapolis.
Frank Cathers, Jr., RPh, is the senior director of strategic services at Precision for Value, where he focuses on segment strategy and brand business planning. He has more than 18 years of experience in pharmacy benefit management, as well as experience in all aspects of pharmacy administration, regulatory compliance, strategic planning, clinical effectiveness, and cost analysis. He received his Bachelor of Science degree in pharmacy from the Philadelphia College of Pharmacy and Science.
Sejal Jonas, Pharm D, is senior advisor of pharmacy benefits and contracting for Precision for Value. She has more than 18 years’ experience as a managed care pharmacy administrator, specializing in sales and account management, clinical program customization, strategic initiatives, and implementation of programs. Sejal received her Bachelor of Science degree in pharmacy and her Doctor of Pharmacy from Philadelphia College of Pharmacy and Science. She is a licensed pharmacist in Pennsylvania and Maryland.
