4 Pharma Manufacturing Trends & Their Implications For Chemical Engineers
By Martina Kotthaus, Ph.D., and Peter Poechlauer, Ph.D., Patheon

Chemical and process engineers who design, develop, and operate pharmaceutical processes and plants can improve their companies’ competitiveness by ensuring they keep track of and pay attention to four key manufacturing trends that currently rise to the top of the industry’s collective demands and needs:
- The increased use of continuous processing
- The rising complexity of chemical structures
- The introduction of new, polymer-based active pharmaceutical ingredients (APIs)
- The need for accelerated process scale-up and process implementation
Increased Use Of Continuous Processing
In recent years, the pharma industry has turned to continuous processing more often in small molecule API manufacturing. This may involve single-unit operations (such as a continuous reaction), single steps (continuous reaction plus work-up), parts of syntheses, or even entire API syntheses. The U.S. Food and Drug Administration (FDA) is supportive of this trend of and emphasizes that the science exists for its success, having written its guidance papers in a way that does not suggest any regulatory hurdles to the implementation of continuous manufacturing. The agency’s website1 describes continuous processing as a “faster, more efficient process,” noting that the FDA “is taking proactive steps to facilitate the drug industry’s implementation of emerging technologies, including continuous manufacturing, to improve product quality and address many of the underlying causes of drug shortages and recalls.” A publication by FDA authors2 asserts that “continuous processing has a great deal of potential to address issues of agility, flexibility, cost, and robustness in the development of pharmaceutical manufacturing processes … The FDA supports the implementation of continuous manufacturing using science — and risk-based approaches.”
Engineers considering the implementation of continuous processing should keep the following in mind:
- Development of continuous processes involves co-development of chemical process and equipment/plant. This requires an intense effort at the beginning of the development phase, including networking within multidisciplinary teams — comprising chemists, process engineers, plant engineers, and process control engineers — to design a production concept that will not suffer from scale-up issues.
- This approach enables demanding chemistry and process safety steps to be implemented on a larger scale, and without the need for process changes. Shorter routes involving highly hazardous, but efficient, reagents can be chosen for scale-up.
- Continuous manufacturing processes create larger data volumes within a short time frame. These data can be evaluated within design of experiments (DoE), a branch of applied statistics dealing with planning, conducting, analyzing, and interpreting controlled tests. DoE is one of the tools used in the quality by design (QbD) approach, which is increasingly being used across drug development and manufacturing to drive robust processes that help deliver both patient safety and the timely and uninterrupted supply of high-quality drug substances and products in a consistent manner. DoE enables models to be developed and built to improve understanding of products and processes, using both statistical and mechanistic approaches.
Rising Complexity Of Chemical Structures
The chemical structures of small molecule APIs are becoming increasingly complex, requiring longer syntheses, often comprising 10 or more steps, and greater effort to develop synthesis and analytical methods. Many of these complex APIs and their intermediates are also highly potent, requiring innovative approaches to ensure safe production, analysis, and storage of these materials.
Selection of the most cost-effective and sustainable chemical route is key to ensuring fast development and scale-up across all project phases — which may require batch capacity ranging anywhere from 1 kilogram to hundreds of tons.
Manufacturing processes for products with exceptionally low by-product content demand exceptional precision in process control and monitoring and require analytical methods that can detect and quantify trace amounts of these by-products.
New, Polymer-Based APIs
Along with small and large molecule APIs, technologies such as emulsion polymerization are being applied more often to make polymer-based APIs — a newer concept in pharmaceutical manufacturing. Analysis of these insoluble materials requires techniques that differ from classical pharmaceutical analytics for traditional small and large molecules.
Since these APIs are typically administered at high daily doses, by-product content must be exceptionally low. This can be achieved using high levels of precision in process control and monitoring, and verified using analytical methods that can detect and quantify trace amounts of by-products. High-volume demand of these APIs begs for large-scale production capacity — of tens to hundreds of tons per year — as well as specialized techniques for cleaning equipment. It has been found that the correlation between process conditions and product properties is much more complex than for small molecule APIs and requires a high level of precision and expertise in manufacturing of these products.
Accelerated Process Scale-Up And Process Implementation
Accelerated process scale-up and process implementation are in increasing demand in the industry, with timelines shortening as the number of products with fast track designation continues to rise (Table 1). This designation — described by one commentator as “fast but flawed”3 — demands increased flexibility in production and more rapid response times to manufacturing requests. Additionally, seamless technology transfers between production plants, or even production sites, may be required to respond to changes in product volume demand at short notice. Issues in fast-track projects require specific attention to process robustness and consistency of product quality to avoid the need for time-consuming repetition of clinical trial phases, production campaigns, or process redesigns.
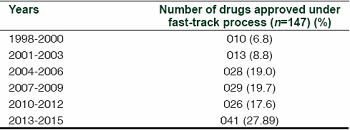
Table 1: Numbers of drugs approved under the FDA fast-track process
As pharma companies are faced with mounting appeals to lower costs and speed up time to market for effective, quality drugs for patients, innovative approaches and partners must be identified to meet these demands and ensure preparedness for fast chemical process and formulation development and the availability of technologies for fast scale-up. This is best accomplished by a highly multidisciplinary approach in which the different disciplines operate in parallel.
Faced with these complexities, pharma companies — and their engineers — have to take an integrated approach to drug substance and drug product development and manufacturing in order to simplify the supply chain from the earliest point in development and ensure quality through every phase of development.
Pharma companies that keep an eye on trends in the industry are best positioned to choose their innovations and end-to-end processes carefully in order to reliably reach their development timelines and deliver consistent high-quality pharmaceuticals to those who need them at an acceptable cost.
References:
- Lee S. Modernizing the Way Drugs Are Made: A Transition to Continuous Manufacturing (May 17, 2017). https://www.fda.gov/Drugs/NewsEvents/ucm557448.htm
- Lee SL, O’Connor TF, Yang X, Cruz CN, Chatterjee S, Madurawe RD, et al. Modernizing Pharmaceutical Manufacturing: from Batch to Continuous Production. Journal of Pharmaceutical Innovation. 2015;10(3):191-910.1007/s12247-015-9215-8 https://doi.org/10.1007/s12247-015-9215-8.
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4936080/
 About The Authors:
About The Authors:
Martina Kotthaus, Ph.D., is the head of research and development for the API business unit at Patheon’s Linz, Austria facility. Kotthaus has been with Patheon since it merged with DSM Fine Chemicals in 2014, and has more than 15 years of experience in the pharmaceutical industry. At Patheon, she is responsible for leading the Linz site’s research and development department. She earned a Ph.D. in organic chemistry from Westfälischen-Wilhelms-Universität, Münster.
 Peter Poechlauer, Ph.D., is the innovation manager for the API business unit at Patheon’s Linz, Austria facility. Poechlauer has been with Patheon since it merged with DSM Fine Chemicals in 2014, and has more than 25 years of experience in pharmaceutical research and development. At Patheon, he is responsible for driving innovation in small molecules manufacturing to develop and implement cleaner, more efficient production processes for compounds, and developing competencies necessary to modernize production methods for clients. He earned a Ph.D. in organic chemistry and pharmaceutical chemistry from Innsbruck University and spent two years completing post-doctorate studies in organic chemistry at the University of Munich.
Peter Poechlauer, Ph.D., is the innovation manager for the API business unit at Patheon’s Linz, Austria facility. Poechlauer has been with Patheon since it merged with DSM Fine Chemicals in 2014, and has more than 25 years of experience in pharmaceutical research and development. At Patheon, he is responsible for driving innovation in small molecules manufacturing to develop and implement cleaner, more efficient production processes for compounds, and developing competencies necessary to modernize production methods for clients. He earned a Ph.D. in organic chemistry and pharmaceutical chemistry from Innsbruck University and spent two years completing post-doctorate studies in organic chemistry at the University of Munich.
