Pharmaceutical Continuous Manufacturing: Content Uniformity With PAT And RTR
By Richard Steiner, practice lead, Pharmatech Associates

In pharmaceutical continuous manufacturing (PCM), technical solutions ensuring critical quality attributes for content uniformity and unit dose exist for online, in-line, and at-line measurements. Process analytical technology (PAT) allows in-line measurement and control of critical process parameters (CPP) that impact a product’s critical quality attributes (CQA).1 When applying quality by design (QbD) principles it is possible to define a process design space for a unit operation and integrate real-time process analytics into pharma and biopharma manufacturing processes. This creates the opportunity for real time release testing (RTRT) to replace conventional end-product testing, potentially saving a drug sponsor facility environmental control costs, inventory carrying costs, and testing costs for product release.
As PAT sensor technology has evolved and matured, so have the approaches to implementation. Today’s best practices simplify the approach to using PAT as part of an RTRT strategy and help drug sponsors avoid complexity that can impact overall equipment effectiveness (OEE).
Control Strategy And PAT
One of the key benefits of PCM is the potential to measure and control the processing of material and ensure that all accepted product is processed within predefined limits. That doesn’t mean all material is within specification, but if process drift is detected the equipment will either compensate for the drift before it is out of specification or reject material that is out of specification. This ensures that the final products manufactured are all within specification. Since the intermediates are transferred from one unit operation to the next without interruption, CQAs can be monitored in real time. This way, any variability of CPPs can be adjusted dynamically according to the level of automation and in-process feedback facilitated by PAT. In-line measurement and control eliminate the uncertainty associated with sampling plans and avoid the risk of Acceptable Quality Level (AQL) and Lot Tolerance Percent Defective (LTPD) frameworks. Coupled with an RTRT strategy, the overall cycle time for manufacturing to product release is much shorter. An example is shown below in Figure 1.
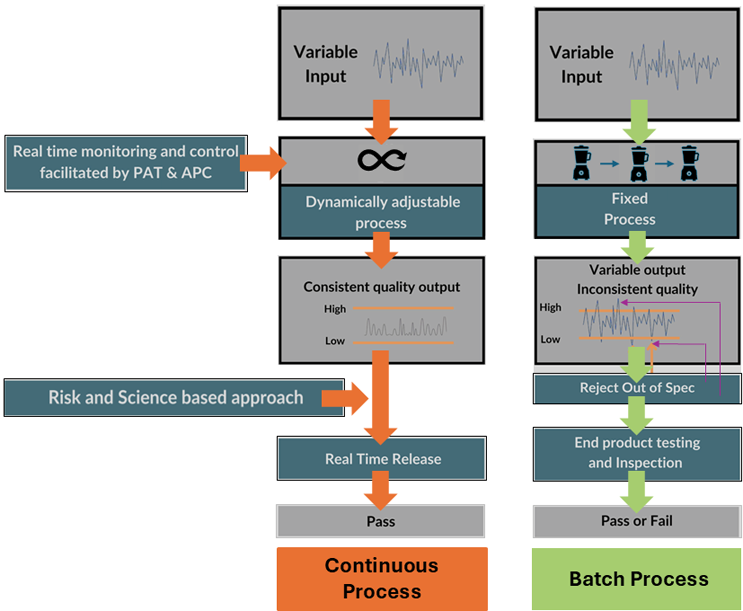
Figure 1: Quality by Inspection versus Quality by Design.
Sensor Technologies And Process Interfaces
Content uniformity (CU) is one of the most important release criteria for solid dosage forms as it ensures that every tablet has the appropriate amount of API in it to be both safe and effective. What in-line PAT technologies are available today to measure and assure CU?
PAT solutions for CU measurement are obtained mainly using in-line spectroscopic sensors such as near infrared (NIR) or Raman. Many mature sensing technologies exist to measure product CQAs within a continuous process — including ultrasound to measure variation in the speed of sound to correlate with physical properties such as powder tensile strength. Laser diffraction can measure particle size distribution, a valuable material property to assess potential sources of deviation in model performance. Laser technology is used to control the powder level in a continuous process. The 3D particle analyzer provides information about the size, shape, and surface morphology. However, today there is not any single integrated analyzer for CU. To implement a true inline RTRT strategy requires building the argument for CU.
Figure 2 lists the typical CQAs for an oral solid dosage (OSD) process relating to product quality. CQAs are generally associated with drug substances, excipients, intermediates (in-process materials), and drug products. NIR is relevant to CU measurement since it is fast enough to allow in-line monitoring. Transmission sensors such as Raman spectroscopy are more accurate but much slower and thus are mainly implemented for at-line and online devices.
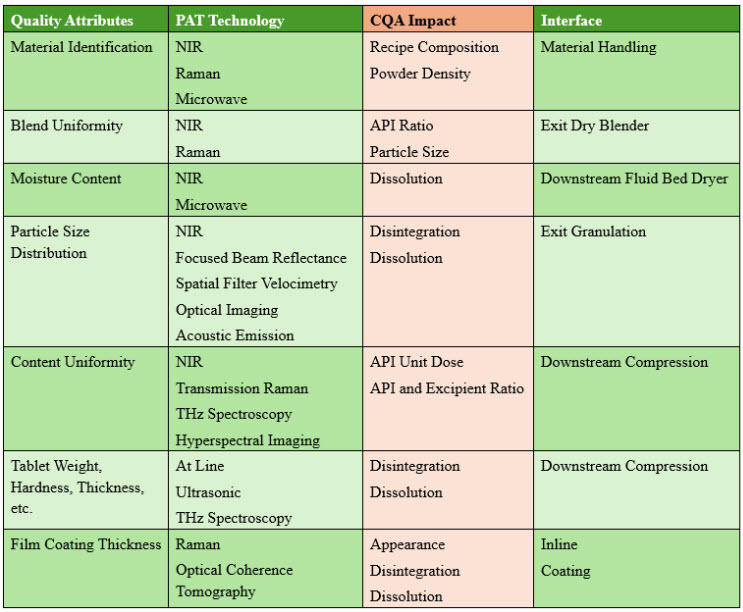
Figure 2: Typical Critical Quality Attributes for Oral Solid Dose Processes.
The pharmaceutical industry is trending toward new drug entities and more potent compounds. Measuring active content in low dose formulations (0.5% to 5% w/w) while using existing spectroscopic PAT (e.g., NIR) in-line, in real-time, while moving formulated powder beds is difficult. High potency active pharmaceutical ingredient (HPAPI), i.e., those in occupational exposure bands (OEB) 4 and 5, containment requirements are increasing because of new and more potent drug candidates. This has significant impact on the process and equipment design to operate safely.
One challenge with an integrated sensor in a powder environment is window fouling, because there is no easy way to clean an integrated sensor during processing. The sensor and window should be positioned in an area where there is constant material turnover, so that the window can be wiped throughout the entire process time. When processing HPAPIs, the actual percentage of API in the formulation is likely to be small, so using a more sensitive but slower sensor technology such as Raman transmission may be more effective. Understanding the spectroscopic requirements of the material being processed and the API itself is a crucial decision component of determining whether an in-line test method for CU should be visible, or if it requires an online or at-line solution.
The most important process interfaces for PCM PAT applications are transfer of intermediates, mechanical supporting of the PAT sensor and electronic interface, sampling ports for a contained sampling operation, and finally a control scheme and discharge port to handle out of specification (OOS) material. According to ICH, the reliability of the entire PCM process is based on the design of the interfaces, not just the function of the sensor itself.2
Current Technical CU Solutions
Several equipment vendors have combined unit operations with highly integrated continuous direct compression (CDC) systems including integrated PAT solutions, mainly to measure blend uniformity (BU). Auxiliary equipment is needed for online, or at-line testing of CU as described in the compendial framework.
- Fette Compacting’s continuous direct compression (CTS) system is an example of a fully embedded PAT (ePAT) system. An integrated NIRS BU (near infrared blending uniformity) sensor and a NIRS reflectance TU (tablet uniformity) sensor in combination with loss-in-weight (LiW) feeders monitor the API concentration of the process in real time for each tablet. Through fast NIRS and the embedded control system, the unit can measure, analyze, and disposition the product on a single tablet basis.
- Pharma Technology, an auxiliary equipment provider, recently launched the CU-120 to be used in-line, online, or at-line. This device has a laser scanner to measure the size of the tablet, a NIR-SRS (spatially resolved spectroscopy) reflection method to measure the API fraction, and a 3D microwave device for the mass measurement. The equipment has a processing rate of approximately 33 tablets a second for a maximum throughput of 120,000 tablets per hour, depending on the API and ingredients.
- Similar technology is used in the UTS Checkmaster from Kraemer. This is an online tablet check device used in tablet compression machines to feed back process parameters to their set points and limits. The UTS NIR IPC from Kraemer comes with a FT-NIR sensor.
- Bruker’s multipurpose analyzer transmission NIR sensors are limited to a capacity of approximately one tablet per minute and thus can take only samples for online testing in a selected frequency.
- The SciYobotic system from Optimal uses a robotic device to pick and transport tablet-by-tablet to a weighing balance for weight and then to the Bruker MP FT-NIR for a transmission test of each tablet’s API fraction, resulting in an CU measurement in accordance with compendial standards.
Each device has its specific operational and installation limits. Good engineering practices will help to select the best fit for purpose. At-line devices work well for process development and predictive model development. Whether or not each of these solutions is capable and ready for the tough environment of a commercial setting with strict containment requirements is determined by risk and OEE assessments as well as experience over time.
Compendial Framework
USP <905> and Ph EUR 2.9.40 and 2.9.47 are the cornerstones of any CU product release testing today. This is because the unit dose or uniformity of a dosage unit is clearly defined and described, as well as the number of samples required for each batch. Today’s compendial framework is based upon traditional batch manufacturing that requires a sampling plan and offline testing. Despite the markedly higher level of measurement and control PCM provides, there is no additional value in sampling and testing product designed for a batch process. Technical solutions exist in the market that would allow 100% testing: we question whether one needs to test 100% of the product considering that PCM offers the potential for Six Sigma process capability.
Content Uniformity Within A RTR Strategy
The control strategy is embedded in the control system of any integrated PCM system. Is it possible to achieve real time release (RTR) when CU is part of the release criteria? Let’s take a closer look at the definition of RTR and real time release testing (RTRT).
According to ICH, RTR is the ability to evaluate and ensure the quality of a process and the final product based on process data that includes a combination of measured material attributes and process controls.1 RTR testing may form the basis, but other aspects should be considered, since the batch release decision must still be made by a qualified person. As described in ICH Q8,1 RTRT can be applied to an output material quality attribute. RTRT is not a regulatory requirement for PCM implementation but, when RTRT is proposed, the associated reference test method should be described. Development of the data collection approach for RTRT should include a risk assessment of how lapses in the data collection (e.g., recalibrating a NIR probe) may affect decisions relating to product quality.
The proposed control strategy should include alternative or additional quality controls to mitigate any risks posed by data lapses. If the results from RTRT fail or are trending toward failure, appropriate investigations should be conducted by the quality organization. A reference to consider is the ICH-Endorsed Guide for ICH Q8/Q9/Q10,1 which suggests implementing process or predictive models to be used as surrogates for traditional release testing methods.
In other words, process models can be used to develop a PCM process or as part of a control strategy for commercial production. Process models may also be used to predict quality attributes in real time, enabling timely process adjustments to maintain a state of control. Developing predictive models for the entire PCM process based on residence time distribution (RTD) is a promising and proven methodology to overcome unnecessary complexity.
Conclusion: The Case For PCM
Today, there are four routes to get to a content uniformity-based release strategy using PCM. First, the most reliable and cost-conscious solution is to develop a predictive model where the PAT sensor monitors verify the CU value through an online or at-line device that checks the API content and weight of each unit dose. The second solution, while costly, is integrating a PAT solution to measure every tablet manufactured as it leaves the outlet of the compression machine. A third solution is the traditional at-line testing method with a typical transmission FT-NIR or Raman system — this is a manual approach that requires time to sample and test material using a sample at least as large as required for a batch process. A fourth solution is to rely on the process capability and the operating characteristics of a given process to calculate the optimal sample size based on stochastics and the operating characteristic curve. Process capability studies have shown that PCM systems can deliver tablet manufacturing on a Six Sigma control level and, therefore, neither a 100% product testing nor a costly in-line PAT solution is required if the necessary controls are in place to control all sources of variation in the process. The benefit of PCM is its process capability and productivity combined with its simplicity and that should not be compromised with overly complex control strategies.
References
- ICH. (2009, August). Pharmaceutical Development Q8 (R2).
- ICH. (2023, January). ICH guideline Q13 on continuous manufacturing of drug substances and drug products.
 About The Author:
About The Author:
Richard Steiner is senior manager, business strategy, at Pharmatech Associates. He has extensive experience in the high-end equipment industry, working in global business networks. He has engineering expertise in continuous processing/manufacturing of oral solid dose pharmaceuticals, hot melt extrusion of pharmaceuticals, and twin- and single-screw extrusion of thermoplastic materials.
