Pharmaceutical Manufacturer Leverages Data And Analytics To Support New Continuous Drug Manufacturing Processes
By Janice Abel
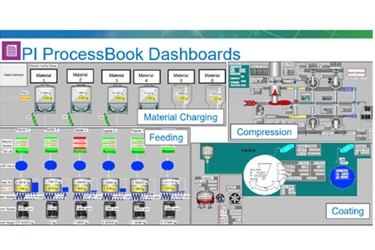
While moving from batch to continuous manufacturing may not be new in many other industries, it is in pharmaceutical and biotech manufacturing. According to Laura Wareham, Senior Scientist, Engineering, at Merck’s manufacturing division, while continuous manufacturing may be well-established in other high-volume industries with well known processes, it is relatively new to the pharma industry. That’s because pharma is a highly regulated, science-based, and largely batch-oriented industry with a large installed base of batch manufacturing equipment.
However, despite the upfront capital expense required to replace legacy equipment and perceived regulatory hurdles, continuous manufacturing can lead to innovative pharma and biotech products and processes and help pharma companies resolve long-standing cost issues related to development and scale-up and delivering high-quality products with flexible batch sizes to meet changing market or customer demands.
Over the past couple of years, the European Medicines Agency and US Food and Drug Administration gave a handful of approvals to pharmaceutical companies to manufacture drug products using continuous manufacturing processes. In February 2019, the FDA released a draft guidance that defined continuous manufacturing as a process in which input materials are continuously fed and transformed within the process and the output materials are continuously removed from the system.
Merck is using the OSIsoft PI System data infrastructure and piloting Seeq’s analytics to optimize its product and processes to support continuous manufacturing.
Get unlimited access to:
Enter your credentials below to log in. Not yet a member of Pharmaceutical Online? Subscribe today.
