Pharmaceutical Transport Validation — Where To Begin?
By Tobias Kuners of Koenders, managing consultant, Tob Management
Contrary to what many people think, the last point of contact between patients and their healthcare providers or the distributors of medicines is not the dispensing pharmacist but instead is the pharmaceutical’s packaging — a function that is recognized as critical. Furthermore, across the full supply chain, packaging safeguards the drug product from external influences and foreign materials. On top of that, during the shelf life of a drug product, the packaging helps ensure claims made on the product’s label can be ascertained through a variety of environmental conditions. To assure that pharmaceutical packaging functions as required, validation needs to be planned and executed before the drug product is available in the marketplace, and it must be continuously monitored during the full life cycle of the drug product. This article provides a road map for transport validation, which is used to qualify packaging for the entire product supply chain. When transport validation is done incompletely, poorly, or not at all, it can result in off-label drugs that are potentially harmful to patients.
distributors of medicines is not the dispensing pharmacist but instead is the pharmaceutical’s packaging — a function that is recognized as critical. Furthermore, across the full supply chain, packaging safeguards the drug product from external influences and foreign materials. On top of that, during the shelf life of a drug product, the packaging helps ensure claims made on the product’s label can be ascertained through a variety of environmental conditions. To assure that pharmaceutical packaging functions as required, validation needs to be planned and executed before the drug product is available in the marketplace, and it must be continuously monitored during the full life cycle of the drug product. This article provides a road map for transport validation, which is used to qualify packaging for the entire product supply chain. When transport validation is done incompletely, poorly, or not at all, it can result in off-label drugs that are potentially harmful to patients.
Designing A Transport Validation Project Plan
Stability studies investigate product characteristics for extended periods, to determine shelf life and required environmental conditions, including temperature and humidity. Storage and transport studies look at the speed at which the product moves and the environmental conditions it will encounter throughout the journey.
Accelerated environmental tests (UV, temperature, moisture), solar radiation tests (discoloration, brittleness, elasticity), and vibration and compression tests are conducted under laboratory conditions before real-life tests in actual circumstances. A transport validation project plan (TVPP) should cover at least three phases: (1) transport validation process design, (2) transport process qualification, and (3) continuous transport monitoring. Such a plan may include tests under laboratory conditions as part of the transport simulation.
For the design, start with the end in mind; the purpose of transport validation is to provide intentional and robust packaging and transport to assure patient safety by delivering the drug product safely. Given the time it requires to plan and execute transport qualification, it is recommended to start the project plan at the clinical stage of the product. Input data for the design of the transport validation project plan includes the drug product label claims, the available stability data, and the existing processes and systems. Where such data is not (yet) available, the validation project plan supports the bracketing of required data. Simulating the transport lanes at laboratory scale can significantly reduce the real-life tests to be conducted. Basic drop-testing, vibration testing, shock impact testing, and accelerated environmental testing with UV-radiation and temperature cycles, under different humidity regimes, can mimic the actual conditions as closely as possible. Results from those laboratory tests provide input to the design and the real-world qualification tests. Transporting product from one place to another can be planned and executed very precisely, but the circumstances under which such a transport unfolds can never be fully planned and controlled. Consequently, we speak of qualification instead of validation.
The design stage of the transport validation project plan covers three elements: content, reach, and means.
Content refers to the type of product being transported, i.e., bulk product (drug substance, drug product), intermediate product, unlabeled finished product, labeled finished product. This distinction is important, as bulk product can be packed differently from intermediate, which can be packaged differently from the finished product. For the content covered in the plan, what is the exposure it will experience during transport? Consider not only the way it is packed, both primarily (in contact with the product) and secondarily, but also the mass packaging (case package, shippers, pallet load). Elements that need to be assessed and valued with a risk-profile for the transport are contact, vibration, temperature, moisture, handling, and any possible other criteria.
Reach covers the farthest distance the product will be transported. Will it be country (local), regional (EU, U.S., Asia Pacific), or worldwide, and does it involve a single or multiple transport routes? For the reach covered in the plan, consider the most severe exposures, distance, handovers, and environmental conditions in transit and during handovers, including temperature ranges expected, relative humidity, time out of refrigeration (TOR), vibration, extended staging, waiting time, etc. With products in early stages of development, stability data may not be fully developed. Don’t consider that a showstopper for testing but take it as a variable and allow the transport validation project plan outcome to feed back the required range that stability testing needs to cover. It may not yet be known what minimum or maximum temperatures the product may be exposed to. However, knowing where the product originates and the farthest it needs to travel will give insight into the lowest or highest temperatures the package can be exposed to. The objective of transport validation is for the product to be unaffected by environmental conditions and to provide valuable data on the expected performance of packaging during the transport.
Means covers the mode of transportation, such as by road, which can differ by car or truck, and by train, sea, and air. Generally, a combination of road, sea, and air may need to be considered. For the means in the plan, consider the roughest means or combination of means, the quality of the roads, vehicles, handovers, and more. The means or a multitude of means have a direct impact on the transport packaging required to avoid an adverse effect on the product under protection. For example, rough handling, substandard trucks, or extreme temperature exposure are but a few of the means that need to be accounted for in testing the packaging and packed product.
Transport Qualification
Transport qualification has to challenge the assumptions outlined in the design, perform tests to the extremes, execute those tests, and perform the actual transport lanes with dummies/placebos, collect the results, and consider backup/fail-safe scenarios. Simulating initial tests in a laboratory setting can reduce costs. In an advanced setting, the data gained from such experiments allows programming the laboratory equipment for the characteristics of the routes it needs to simulate, reducing the necessity for extensive real-world testing.
A high-level process map could have the following main steps: (1) produce, (2) pack, (3) store, (4) prepare (for shipment), (5) stage, (6) load, (7) ship, (8) receive, (9) unload, and (10) store, where steps 4 through 9 can repeat depending on inbound or multiple outbound storage locations.

Transport qualification is the actual execution of the approved transport validation project plan. A positive outcome from the qualification qualifies the tested transport packaging and routes.
Continuous Monitoring Through KPIs
The final stage is continuous monitoring, to assure product protection and to assess the route and conditions, allowing for data-driven decisions to drive desired or required changes. Continuous transport monitoring requires a process to be stable and capable. Stable suggests that the average and variation of any measure monitored over time follows a straight path and is within an acceptable range.
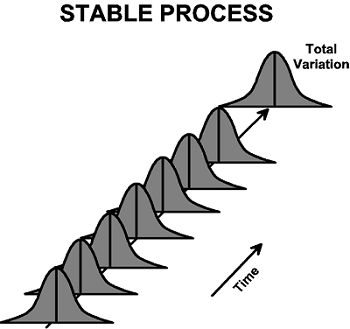
Capable suggests that the averages and variation of such measures monitored over time stay within the specification limits.
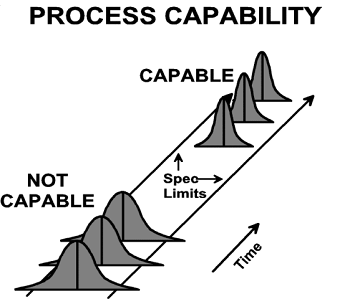
To monitor whether the transport is efficient (doing the right things) and effective (doing the things right), you will need to establish metrics or key performance indicators (KPIs), design a dashboard to report those KPIs, and agree upon a reporting frequency. A dashboard with a complete and comprehensive overview, showing enough, but not too much, information is more likely to get the focus and attention required to drive improvements. Appropriately selecting the few true KPIs is only possible with in-depth knowledge and understanding of the complete route and all the critical steps within it.
For a KPI to measure effectiveness in a process, it needs to address an activity between two process steps (doing the things right – delivering what the following process step requires). To measure the efficiency, for that same process step, the critical activity within the process step needs to be addressed. With diligence, identifying one key activity between each major process step and one within such process step limits the total number of KPIs. As KPIs represent a collection of performance indicators, a deviation toward (in control) or outside (out of control) the specification limits triggers detailing the underlying performance indicators for the root cause.
Conclusion
A transport validation project plan may look like a lot of work, but, as discussed, with proper planning and meticulous execution, a cost-effective, comprehensive process can be designed, executed, and monitored.
This article does not address the cost of designing, executing, and monitoring shipments. Compare the costs of a TVPP with the loss of a single shipment of the product as a result of incomplete design or incorrect execution of transport. Existing data on transport losses is sporadic and fragmented, as pharmaceutical companies do not readily share their experiences with transport losses. Transports are typically by the pallet or truckload. With several hundreds or even thousands of doses of drugs per pallet – aside from the regulatory requirements to perform transport validation – the cost of transport validation is small compared to the loss of a shipment.
About The Author:
 Tobias Kuners of Koenders is managing consultant at Tob Management, a Netherlands-based consulting firm providing business and technical expertise to the pharmaceutical, biotech, medical device, and FSMP (food for special medical purpose) industries. He has worked at Biogen, Ipsen, Patheon, and Danone, among other companies, gaining hands-on experience in engineering services, equipment qualification, maintenance, supply chain, warehousing, and distribution. He has led design, engineering, and construction of multiple facilities to meet cGMP requirements. He performs audits, identifies gaps, and develops remediation plans, working within organizations to assist with deviation investigations and CAPA implementation and to develop scientific, risk-based solutions. You can contact him at connect2tobias@outlook.com.
Tobias Kuners of Koenders is managing consultant at Tob Management, a Netherlands-based consulting firm providing business and technical expertise to the pharmaceutical, biotech, medical device, and FSMP (food for special medical purpose) industries. He has worked at Biogen, Ipsen, Patheon, and Danone, among other companies, gaining hands-on experience in engineering services, equipment qualification, maintenance, supply chain, warehousing, and distribution. He has led design, engineering, and construction of multiple facilities to meet cGMP requirements. He performs audits, identifies gaps, and develops remediation plans, working within organizations to assist with deviation investigations and CAPA implementation and to develop scientific, risk-based solutions. You can contact him at connect2tobias@outlook.com.
