Polymorph Prediction in Drug Development
By Katriona Knapman
Molecular Simulations
Polymorphism, the ability of a molecule to crystallize into more than one different crystal structure, affects the taste of chocolate, the color of Ferraris, the strength of drugs, and can determine whether a competitor can steal your new product—yet experimental polymorph prediction is painstaking, and always leaves scientists wondering whether they have found all of the possibilities. Now new polymorph prediction and powder diffraction software offer chemists a helping hand in determining the possible polymorphs of organic compounds.
This fictional case study by Katriona Knapman of Molecular Simulations illustrates the potential importance of polymorphs in drug discovery and new product introductions.
The Importance of Polymorphism
Identifying Polymorphs
References
In January 1998, the worldwide pharmaceuticals manufacturer Drugs Inc. released their new WonderDrug on the US market. Hailed as the new leader for over-the-counter pain control, the drug was successfully launched and enjoyed considerable market growth during its first six months on the market.
Drugs Inc. were on their way to recouping the 300 million dollars that they had spent on the research and development of the drug over the last ten years. Their forecasts predicted that with sales continuing at the current level, it would take five years for them to recoup their investment—faster than average for the industry.
In July 1998, Pills PLC released a rival product FantasticDrug. Claiming similar properties to WonderDrug, it was sold at two thirds of the price. Drugs Inc.'s hold on the market was gradually eroded, as consumers turned to Pills PLC's cheaper alternative. Drugs Inc. were forced to cut their price, but Pills PLC retaliated by cutting prices still further until Drugs Inc. were no longer willing to follow.
Dr Andrews and Dr Brown, who were working for Drugs Inc. at the time, were given the task of analyzing Pills PLC's new FantasticDrug. It was as they suspected. FantasticDrug was nothing other than a different crystalline form of WonderDrug.
Patents on drug molecules are taken out on their crystalline forms, in addition to the molecules themselves. Believing there to be only two crystalline forms of WonderDrug, Drugs Inc. had only covered themselves for those forms. By finding a new polymorph, Pills PLC had effectively stolen Drugs Inc.'s years of research and investment, and Drugs Inc had no legal comeback.
The Importance of Polymorphism (Back to Top)
Polymorphism is the ability of a molecule to crystallize into more than one different crystal structure. Different polymorphs have different arrangements of atoms within the unit cell, and this can have a profound effect on the properties of the final crystallized compound.
The chocolate that has gone soft on the back shelf of your car shows a common example of the effects of polymorphism. When it resets it has white patches on it, it no longer melts in your mouth, and it certainly doesn't taste as good as it should. This is because chocolate has many possible polymorphs. There is only one of them that tastes good, and this is the one that is created under carefully controlled factory conditions. Unfortunately these are not the same as the conditions in your car, and when chocolate sets in your car it sets into another polymorph. Polymorphism is responsible for the bad tasting chocolate.
It is not only chocolate that is susceptible to the unwanted effects of polymorphism. The color of dyes can be affected by the polymorph of the pigment. Try telling Coca-Cola why their cans aren't the corporate shade of red, or the new owner of a red Ferrari why his car doesn't match last year's model!
Quinacridone is the parent compound of one of the most important classes of organic pigments and is known to exhibit three polymorphic forms, each with a different shade of red. Quinacridone shows superior performance due to its outstanding light fastness, weather resistance, and thermal stability. Red Ferraris are painted with quinacridone. The picture shows the gamma polymorph of quinacridone.
More seriously, the action of a drug can be affected by the polymorphism of the drug molecules. Different polymorphs can have different rates of uptake in the body leading to lower or higher biological activity than desired. In extreme cases an undesired polymorph can even be toxic.
Last year, Abbott Laboratories had to withdraw their HIV protease inhibitor drug, Norvir, from the market due to the fact that an unwanted polymorph of the drug had been produced during manufacturing. Instead of tablets consisting entirely of the known polymorph of the drug, a new polymorph named Form II ritonavir, was crystallizing in the capsules. This form had a different dissolution rate to the known polymorph, so the bioavailability of the final capsule was affected.
Production of the capsules had to stop immediately until the problems with the manufacturing process could be rectified. Patients using the drug had to rely on a liquid form until manufacturing could be resumed. Abbott could not risk supplying their patients with a polymorph that differed in bioavailability to the desired polymorph. [1]
The problems encountered by Drugs Inc and Pills PLC in the introductory example were also due to polymorphism. In this case, the problem arose through the fact that the crystallized forms of drugs that are patented, in addition to the molecules themselves. When Drugs Inc. filed their patents on WonderDrug, Pills PLC immediately started work on finding a new unpatented polymorph. Pills PLC had been fortunate to find an unpatented polymorph with very similar biological activity to WonderDrug (and to win the associated court cases when they filed their patent and registered the drug with the FDA). This small amount of effort expended on finding a new polymorph, compared to the years of research and development put in by Drugs Inc. meant that FantasticDrug could easily undercut the prices charged for WonderDrug.
The potential effects of on biological activity and the strong competition in the drugs sector makes it vital to identify, analyze, and patent every polymorph of a new drug molecule. However, finding all possible polymorphs is not as easy as it sounds.
Identifying Polymorphs (Back to Top)
Traditionally, different polymorphic structures can only be found by creating them in the lab. Different polymorphs may be produced under different conditions, and the chemist must try to vary conditions in order to achieve as many different polymorphs as possible.
The resulting crystalline structures can then be analyzed using X-ray diffraction. If a pure single crystal can be grown, then single crystal X-ray diffraction is the best way to get high quality data about the crystalline structure. However, it is often difficult and time-consuming to grow crystals large enough to be examined using single crystal X-ray diffraction. In such cases only a powder can be crystallized, and the resulting X-ray powder diffraction pattern is difficult to interpret.
The whole process is difficult and time-consuming. Success depends not only on the skill of the chemists in interpreting the X-ray diffraction patterns, but also on whether they have happened to crystallize all of the polymorphic forms of the molecule. There is no guarantee that they have found every polymorph.
Recent work from Dr Heinrich Karfunkel and Molecular Simulations Inc. (MSI) has meant that there is now a great deal of help available to chemists who wish to determine all of the polymorphic forms of a molecule.
C2.Polymorph uses a Monte Carlo simulated annealing algorithm to simulate thousands of possible crystal packing alternatives for the molecule. Each unique structure is then subjected to a lattice energy minimization for all inter- and intra-molecular degrees of freedom. The structures are then ranked in order of stability, and the lowest-energy (most stable) structures are identified as potential polymorphs. For simple molecules C2.Polymorph can predict the crystal structures of possible polymorphs without any prior knowledge other than the molecular formula.
MSI's C2.PowderSolve software also lends a hand in the process by helping scientists to interpret powder diffraction data, and therefore determine crystal structures more easily.
Scientists at Novartis recently proposed 4-Amidinoindanone guanylhydrazone as a selective inhibitor of S-adenosylmethionine decarboxylase making it a potential anti-cancer drug [2]. Two anhydrous polymorphs of this compound were known to exist, but only one crystal structure had been determined experimentally, as suitable single crystals of the other polymorph could not be grown. Dr Heinrich Karfunkel and co-workers at Novartis [3] were able to determine the unknown polymorphic form using low-quality powder diffraction data and the MSI Polymorph Predictor.
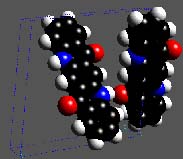
The predicted structure of the previously unknown polymorph of 4-Amidino-indanone guanyl-hydrazone. [3, 4]
This work shows that it is possible to determine by computational methods in a routine manner the crystal structure of a molecule with moderate flexibility. However, the current simulation technology in polymorph prediction means that ab initio screening is only viable for non-ionic, rigid molecules. For more complex systems the method is very useful for generating plausible crystal structures, but it is not accurate enough to determine which of these possible structures can actually be crystallized. [4]
Drugs Inc are now using C2.Polymorph and C2.PowderSolve. They are due to launch SuperDrug in Europe in October 1999, and this time they are confident that all of the polymorphs have been identified. This time any competition from Pills PLC will be from a genuine rival drug, and not from a polymorph of SuperDrug.
- More information on Abbot's withdrawal of the capsule form of Norvir.
- Stanek J., Caravatti G., Frei J., Furet P., Mett H., Schneider P.,Regenass U., J. Med. Chem., 36, 2168, (1993).
- Karfunkel H.R., Wu Z.J., Burkhard A., Rihs G., Sinnreich D., Bürger H.M., and Stanek. J., Acta Cryst., B52, 555, (1996).
- "Computer Simulation to Predict Possible Crystal Polymorphs" P. Verwer and F.J.J. Leusen, in Reviews in Computational Chemistry, K.B. Lipkowitz and D.B. Boyd, Eds., Wiley-VCH:New York, Volume 12, pp.327-365 (1998).
For more information: Katriona Knapman, Senior Marketing Specialist, Molecular Simulations Inc., 230/250 The Quorum, Barnwell Rd., Cambridge CB5 8RE, UK. Tel: +44 1223 413300. Fax: +44 1223 413301.
