Powering Production Of Viral Vector-Based Therapies

Gene therapies hold immense potential to provide long-lasting treatments for a wide range of diseases. At Cytiva, we offer GMP-compliant, end-to-end manufacturing solutions designed to support the development and commercialization of advanced medicines across multiple modalities – including AAV, adenovirus (AV), lentivirus (LV), plasmid DNA (pDNA) and exosomes. Our dependable suite of technologies and expertise ensures scalability, quality, and regulatory confidence at every stage of your journey.
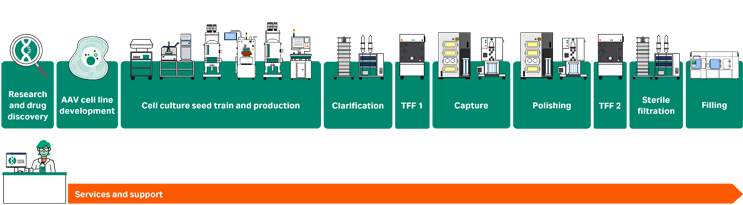
Comprehensive solutions for every workflow step
Cell line development: with our suite of cell lines, you’re all set! Choose from transient, packaging, or stable producer cell lines to fit your production volume needs.
Upstream: develop scalable and robust processes for adherent or suspension cells with high-productivity media and single-use bioreactor platforms – from bench top to pilot to large scale (up to 500 m2 or 2000 L).
Downstream: maximize recovery and purity for all filtration and chromatography steps. Our polishing protocol fully separates empty and full capsids for multiple AAV serotypes. To minimize experiments and gain speed, consider mechanistic modeling.
Empowering your journey to commercialization
Early-stage support
Navigate the early stages of advanced therapy development with the help of our collaborative process development solutions and guidance.
Scale-up expertise
Transition from clinical trials to commercialization with our expertise in scaling up manufacturing processes.
Regulatory compliance
Achieve successful product approvals and launches with support from our deep understanding of regulations and quality requirements.
