Regulations And Guidelines For Mapping Temperature And Humidity Of Pharmaceuticals
By Bruce McDuffee
What Guidelines are available that cover Environmental Monitoring/ Mapping In Life Sciences?
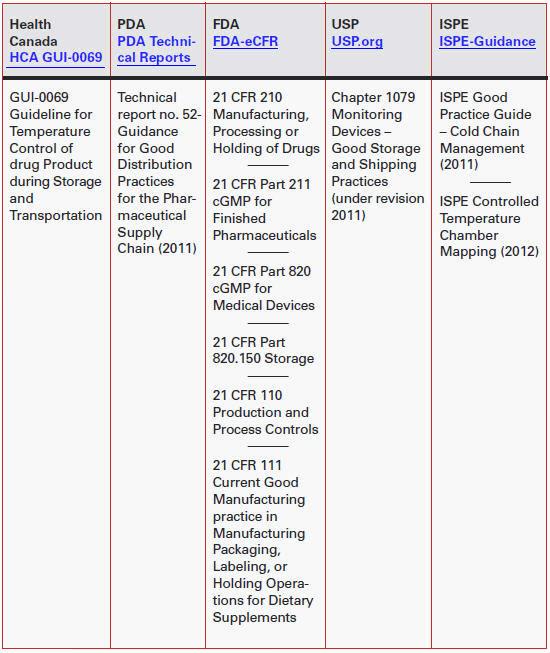
There are several guidelines from a myriad of agencies around the world. The table at right lists several agencies and related documents pertaining to the pharmaceutical industry in North America.
Are there any guidelines that tell me how to environmentally map a chamber or storage area?
There are many guidelines currently available but these guidelines do not instruct one on how to conduct a temperature or humidity mapping study. The guidelines tell you that you need to conduct a mapping study, but not how to actually accomplish the mapping study.
What agencies and guidelines apply to areas outside of North America?
There are many guidelines for many different regions of the world. Some of these can be a good resource even if they may not apply to your geographic region or industry. If your company ships to regions outside of your own, it is a good idea to research and understand the governing regulations and guidelines in those regions.
The World Health Organization has an excellent guideline for mapping storage areas. It provides step-by- step information for a successful mapping project. Some countries refer to the WHO document as the basis for their own regulations. The guideline is the:
Technical supplement to WHO Technical Report Series, No. 961, 2011 Annex 9: Model guidance for the storage and transport of time and temperature–sensitive pharmaceutical products WHO.org Supplement 8: Temperature mapping of storage areas
Why are there so many different regulations?
The regulations have grown organically over time and sometimes can cause confusion. Growth of these seemingly competing regulations is due to factors such as industry needs, different application needs, and regional or professional requirements. In most cases, the guidelines include guidance for mapping in storage, production and cold chain applications. Global harmonization of some of these regulations is an ongoing discussion and will continue over many years. Since most of the guidelines simply state the need for environmental mapping and monitoring they are near equal in their requirements.
Is there one Regulation that supersedes all others?
No single regulation or guidance document supersedes all others. The main considerations for your mapping study will be determined by your specific product, application and industry.
Wouldn’t the FDA guidelines be the best to use since they should be the most stringent?
Not necessarily. The FDA guidelines tell very little about how to conduct a mapping study or how to environmentally monitor an area. FDA regulations simply state that a mapping study is needed.
Some of these guidelines bring up continuous environmental monitoring. Will I be required to do that?
In storage areas a continuous monitoring system is always a good idea. Your mapping study will determine the hot and cold zones for “worst case” sensor placement. These worst case locations should be considered when installing a permanent monitoring system. The number of sensors used for a permanent system will be greatly reduced and may require only a few sensors once the problem areas have been determined through the mapping study. A continuous monitoring system will bring peace of mind that your product components, manufacturing space, or storage space is kept within specified environmental conditions.
For equipment chambers the mapping study is normally conducted to prove that the existing equipment’s control system is functioning properly. The equipment may already have the necessary sensors and documentation system required and therefore a continuously monitoring system may be redundant. However, in some cases an additional monitoring system for equipment can enhance record keeping and add as a secondary verification of the equipment sensors.
With all of these guideline there must be something more specific on how to prepare and conduct a humidity and temperature mapping?
For storage areas the WHO guideline listed above is an excellent resource.
For environmental chambers and other equipment, you may want to explore the “ISPE Controlled Temperature Chamber Mapping (2012)”. This does provide some very good concepts for mapping (i.e. a minimum of 9 probes for chambers less than 2 meters cubed, and minimum 15 probes for anything above 2 meters cubed to 20 meters cubed).
All of these documents are there for guidance, but remember that they are not intended to replace any existing guidelines or to inhibit better practices. Guidelines are a great starting point and provide direction, but solid science with solid data and documentation will help greatly to deliver a successful mapping project.
