Simulations Plus Forges Consortium for Dissolution Prediction
Why Dissolution Software?
Formulation is vital since approximately 80% of all drugs are dosed orally. Dissolution prediction software helps development teams determine the optimal formulation for a given drug dose. Software takes into account single- and multi-layer tablets, capsules, coatings, excipients, processing variables, and experimental conditions.
During late-stage clinical trials but before NDA submission, pharmaceutical companies must finalize a formulation. Software developed through the consortium is expected to reduce the need for at least some time-consuming, expensive laboratory studies, thereby accelerating time to market for both new drugs and new formulations of existing products.
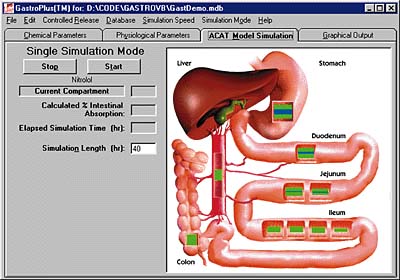
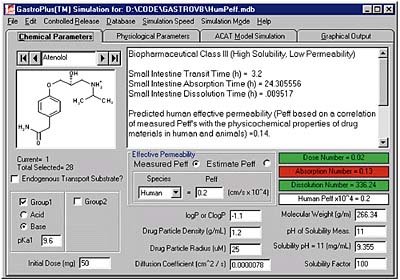
Following its successful introduction of GastroPlus, Simulations Plus hopes to develop a similar product for drug dissolution studies. GastroPlus examines where in the digestive tract (above) a particular drug structure (below) will be absorbed.
"Formulation scientists face a complex problem in trying to find the optimal design for a particular drug dose," said Michael Bolger, director of life sciences for Simulations Plus. "Current methods consist of iterative ‘cut and try' methods in which the formulation designer guesses at a design, makes a batch of tablets or capsules using that design, runs laboratory experiments to measure various properties of the design, and analyzes the results. More often than not, the design needs to be changed, and the process is repeated many times to achieve an acceptable formulation. Each iteration takes time and costs money"
The new software leverages investment Simulations Plus has made in its current suite of drug development products, GastroPlus and QMPRPlus. Much of the underlying code from those products will be directly applied to the firm's new program, which will be called DDDPlus (Dose Disintegration and Dissolution Plus).
Similations Plus likens the problems faced by drug development scientists with issues facing the aerospace industry 20 years ago for solid rocket propellant formulation design. The company believes that with today's much more powerful computational tools optimal formulations can be achieved—not perfectly, perhaps, but well enough to have tremendous economic value to pharmaceutical companies.
For more information: Michael Bolger, Simulations Plus Inc., 1220 West Ave. J, Lancaster, CA 93534-2902. Tel: 661-723-7723. Fax: 661-723-5524.
Angelo DePalma
