StreetSideInvestor interviews Medarex CEO Donald Drakeman

We're speaking with Donald Drakeman, CEO of Medarex, a biotechnology company that develops therapeutic products for life-threatening and debilitating diseases based on proprietary technology in the field of monoclonal antibodies.
By Todd Jerles
StreetSideInvestor
StreetSideInvestor: What does Medarex do?
Drakeman: We use monoclonal antibody technology to turn genomic discoveries into new treatments for cancer and other life threatening and seriously debilitating diseases.
StreetSideInvestor: One of your most interesting and successful technologies is the HuMab-Mouse. What exactly is the "mouse," and how can it be used for medical gain?
Drakeman: Antibodies are the body's first line of defense against disease. Unfortunately, our bodies don't always make antibodies when they should. For example, cancer is a disease of your own cells, and the body is not good at making antibodies against cancer. We can make completely human antibodies against cancers and other diseases by using our HuMab-Mouse. This is a mouse that has been genetically engineered to have certain human genes instead of mouse genes. In particular, the mouse genes responsible for making antibodies have been knocked out, or eliminated, and they've been replaced by human genes for making antibodies. So, when a mouse responds to a cancer tissue that we put into the mouse, the mouse will make human antibodies like the ones you and I might make, and those antibodies can be used to treat human patients without any concern about the antibodies looking foreign or strange to the patients' immune systems.
 StreetSideInvestor: Please elaborate on the numerous corporate collaborations that Medarex maintains centering around the HuMab-Mouse technology, and do you expect any new partnerships to arise in the near future?
StreetSideInvestor: Please elaborate on the numerous corporate collaborations that Medarex maintains centering around the HuMab-Mouse technology, and do you expect any new partnerships to arise in the near future?
Drakeman: Because our technology is the fastest and the most efficient way to move from genomic discovery to new treatments for disease, our HuMab-Mice and other antibody technologies are very attractive to many, many companies in the pharmaceutical and biotech industries. Right now we have 22 corporate partners working with us on this technology including many of the largest pharmaceutical and biotech companies like Novartis, Johnson & Johnson, Bristol-Myers, Amgen, Immunex, and others. We are in the middle of discussions right now with a long list of potential partners, and we expect to be announcing additional partnerships in the near term, in the mid term, and the long term. This is a technology with a great deal of potential, and I think people are recognizing that and want to take advantage of it for their own product development programs.
StreetSideInvestor: How does your recently completed T-12 Development program serve to more quickly and cost-effectively bring drugs to clinical trial?
Drakeman: Traditionally it has taken the pharmaceutical industry 5-6 years and $20-30 million to move from having an interesting disease target to actually launching human clinical trials for a new therapeutic product to treat that disease. So the target-to-trial timeframe is quite a long time, taking 5-6 years. Our T-12 development program means target-to-trial in 12 months, and we have recently taken a fully human antibody from disease target to clinical trials in one year. This speed and efficiency allows us to take advantage of the new medical opportunities being presented by the genomic revolution in an unprecedented way, and that will allow both Medarex and our partners to develop new products faster than has been possible in the past.
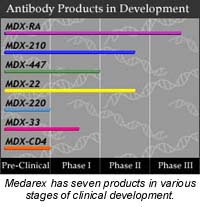
StreetSideInvestor: What other technologies and products are currently in the works at Medarex, and what are the clinical statuses of these products?
Drakeman: We've been in the monoclonal antibody business for about 10 or 11 years now, and before the development of the fully human antibody technology, we were working with partially human antibodies which were the cutting-edge technology of the time. We have a number of products in clinical trials all the way from a Phase I, which is the first level of testing, to Phase III, which is the last clinical trial before filing with the FDA to begin commercial sales of the product. So we're in a number of disease areas ranging from cancer to autoimmune disease and look for these products to be moving forward while we inaugurate a number of new trials each year for our new fully human antibodies.
StreetSideInvestor: At what point do you foresee Medarex achieving a profitable state?
Drakeman: The Wall Street analysts put those dates anywhere between 2 and 4 years from now. Our focus right now at Medarex is to make a significant investment in R&D so that when we become profitable, we become extremely profitable based on a broad pipeline of important new products.
StreetSideInvestor: Finally, if there was one thing you could leave with our readers to think about when Medarex comes to mind, what would it be?
Drakeman: Genomic discovery is changing medicine the way the Internet changed communications. Medarex is in the position to be the fastest and the most efficient developer of new genomic medicine thanks to its human antibody technology.
Todd Jerles of StreetSideInvestor does not own or short individual stocks. The information in this column under no circumstances serves as a recommendation to buy or sell stocks. Visit StreetSideInvestor at www.streetsideinvestor.com.
