Survey: Electronic data capture speeds drugs to market
The results of CB Technologies' 2000 survey clearly indicate the growing acceptance and use of electronic data capture (EDC) technology for clinical trials. The survey shows that pharmaceutical, biotechnology, medical device and contract research organizations are seeing the benefits of EDC as both a cost-saving technology and as a technology that helps to generate revenues by getting products to market faster.
The companies responding run multiple trials per year: 37% are conducting more than 50 trials while just 11% are conducting fewer than 10 per year. 64% of responding companies are currently using or have used EDC with 63% having positive or neutral experiences with the technology. 35% plan to use EDC within the next 3-18 months while only 6% say they have no current plans to use EDC.
Study Methodology
CB Technologies, Inc., the premier provider of technology tools and services to the life science industries, initiated its second annual industry survey on the use of clinical electronic data capture systems in the fourth quarter of 2000.Survey data was collected through hard copy surveys mailed to industry members and through phone interviews conducted in conjunction with the University of the Sciences in Philadelphia. 1200 pharmaceutical corporations, biotechnology firms, clinical research organizations and academic research centers were surveyed with 109 respondents from seven countries, including Canada, France, Germany, Italy, Sweden, the United Kingdom, and the United States.
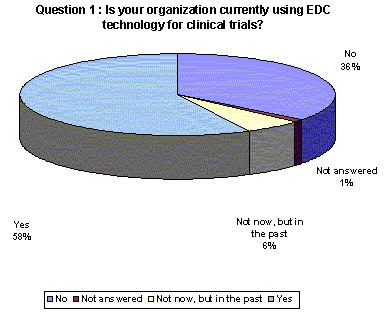
Nearly two-thirds of the responding companies are using or have used EDC technology, while just over one-third have not yet implemented an EDC system for clinical trials.(Back to top)
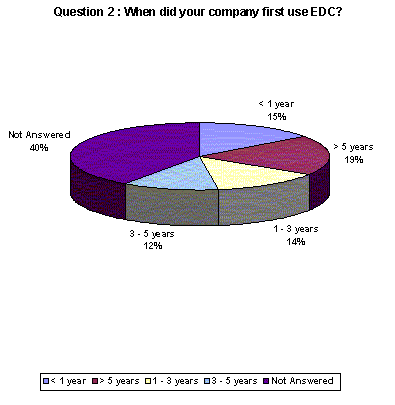
15% of respondents have been using EDC for less than a year. 31% have been using the technology for more than three years. 40% of respondents chose not to answer.
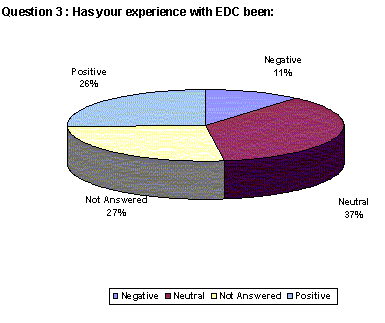
Overwhelmingly, companies are having positive or neutral experiences with EDC (63%). Only 11% indicate a negative experience.(Back to top)
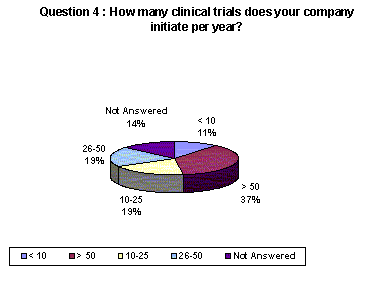
Companies responding to the survey conduct many clinical trials per year. 37% of respondents conduct more than 50 trials and an additional 38% initiate between 10-50 trials per year.
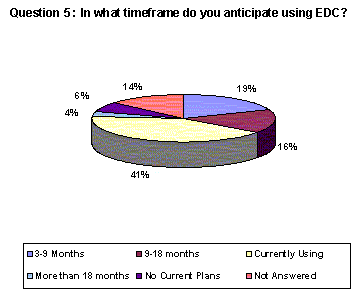
While 41% of responding companies indicated that they are currently using EDC, 35% responded that they intend to use EDC for trials within the next 18 months.(Back to top)
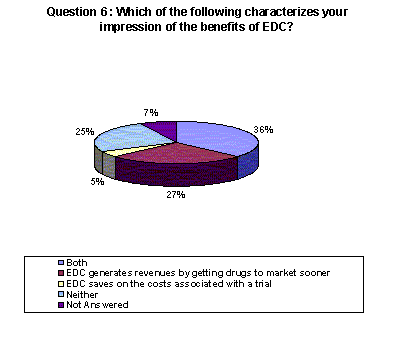
68% of respondents indicated that EDC either generates revenue by getting drugs to market faster (27%), saves on the costs associated with a trial (5%) or both (36%).
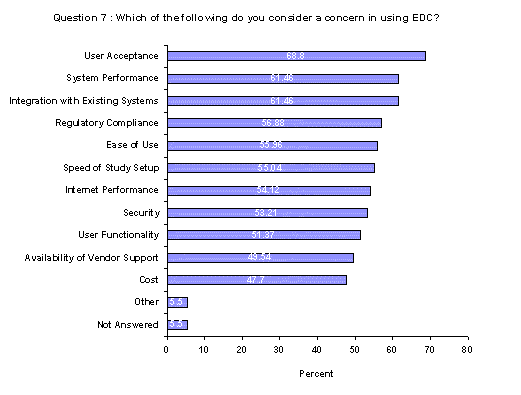
User acceptance tops the list of concerns for companies implementing EDC with the greatest number of respondents identifying this issue (69%). System performance and Integration with existing systems followed with 61% listing each of these. For this question, respondents were asked to check all that apply.(Back to top)
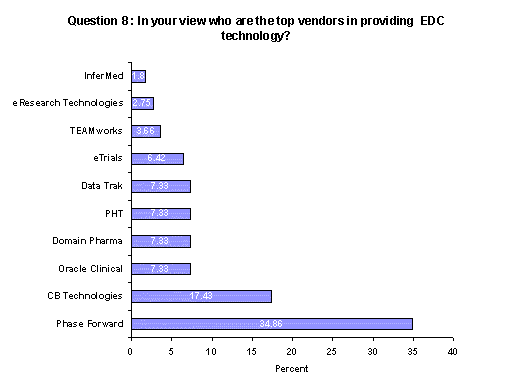
In an open-ended question, respondents were asked to name which companies they considered to be the top five EDC vendors. It is important to note that nearly 40% of respondents did not answer the question. Of those who answered, approximately 34% mentioned Phase Forward as among the top five vendors; 17% named CB Technologies and 7% each mentioned Oracle, Domain Pharma, PHT and DataTrak.
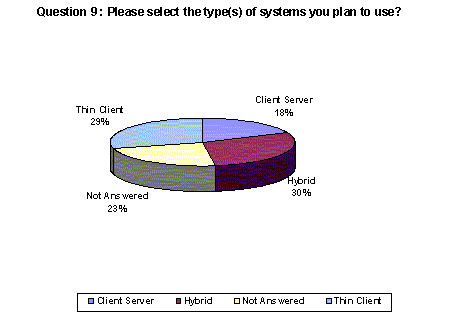
A slightly higher number of respondents (30%) indicated that they plan to use a hybrid system for data capture with 29% listing thin client. Nearly one-fourth of respondents chose not to answer this question.(Back to top)
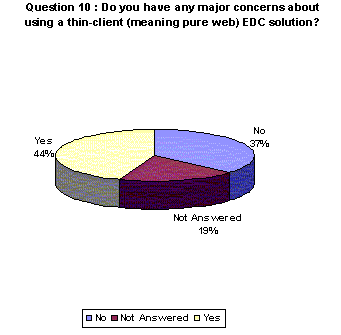
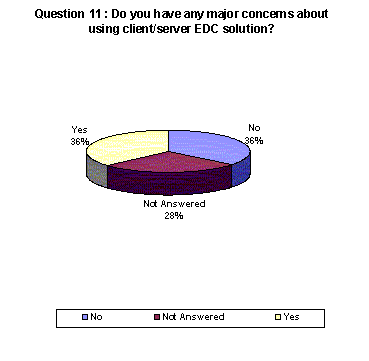
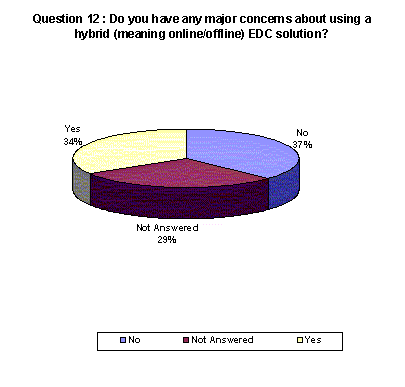
A higher percentage of respondents acknowledged having concerns about using a thin-client system over other systems.
- 44% say they have concerns with thin client. 19% chose not to answer.
- 36% who have concerns regarding client/server. 28% chose not to answer.
- 34% who have concerns with a hybrid system. 29% chose not to answer.(Back to top)
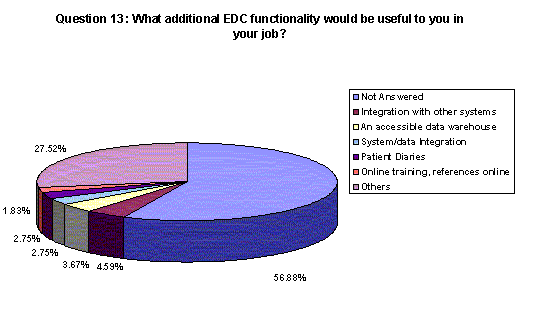
In an open-ended question, companies were asked to identify additional functionality that would be helpful to them in an EDC system. While 57% did not answer the question, integration with other systems was most often noted by those who did answer (5%).
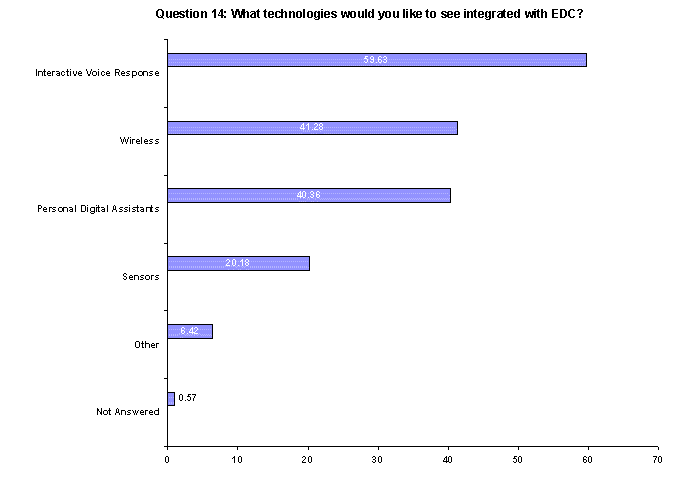
Companies are looking for integrated technology solutions. Nearly 60% of respondents want interactive voice response integrated with EDC, 41% are looking for wireless capability and another 40% want personal digital assistants. For this question, companies were allowed to give multiple responses.(Back to top)
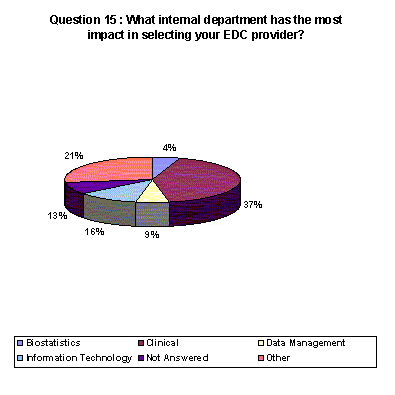
37% of respondents say that their clinical divisions have the most impact in selecting the EDC provider while 15% list the information technology department as having the most impact.
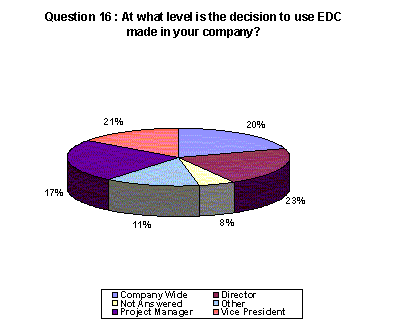
Regarding who makes the decision about EDC, respondents were nearly evenly divided with 21% indicating that the decision is made at the vice president level and 23% listing the decision maker at the director level. 20% indicated that it is a company-wide decision.(Back to top)
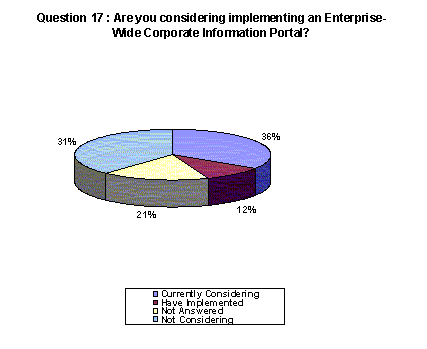
36% of responding companies are considering an enterprise-wide corporate portal while 31% are not currently considering this. 21% chose not to answer and 12% have already implemented a portal.
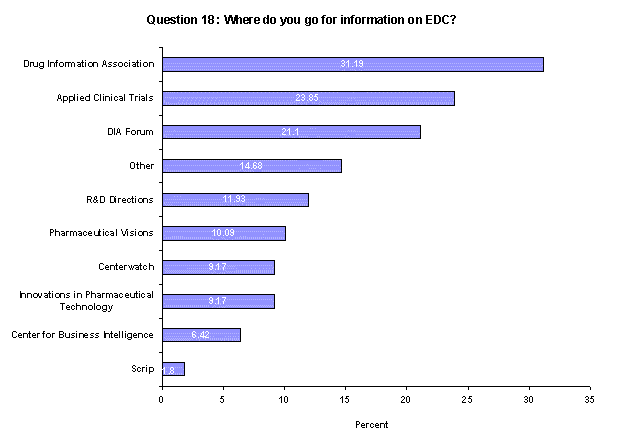
When looking for information on EDC, respondents list Drug Information Association (DIA) events as the place they most often go (31%). Nearly 24% turn to the publication Applied Clinical Trials and 21% use the DIA Forum to find information.(Back to top)
About the Study Sponsor
CB Technologies™ is the premier provider of Electronic Clinical Intelligence™ tools and services to the life science industries. CB's MetaTrial™ provides the most flexible EDC platform available and has become the gold standard solution for reliable, secure, easy-to-use EDC. With hybrid and thin-client architectures that work in concert, clients can select the solution that best suits each site within a study. CB's myMetaTrial portal is a powerful web portal essential for tracking and managing clinical studies.
CB's complementary suite of services and training provide for a smooth implementation of the MetaTrial EDC system.
For more information: CB Technologies, Inc., 350 Eagleview Boulevard, Exton, PA 19341. Phone 610.280.7400. Email: info@cbtech.com.(Back to top)
Source: CB Technologies, Inc.
Subscribe to our free e-mail newsletter.
Click for a free Buyer's Guide listing.
