Targeting SORT1 To Unlock The Potential Of Peptide–Drug Conjugates In Oncology
By Christian Marsolais, Ph.D., and Michel Demeule, Ph.D., Theratechnologies
The landscape of personalized cancer treatment is rapidly shifting to the adoption of advanced therapies such as antibody–drug conjugates (ADCs) and immune checkpoint inhibitors. Yet despite such advances, there remains a demand for precisely targeted therapies with more favorable tolerability profiles and for improved delivery systems for safe and effective combination treatments. Peptide–drug conjugates (PDCs) — therapies that generally employ a cleavable or non-cleavable linker to attach a receptor-targeting peptide to a drug payload — have emerged as a potentially viable modality for addressing that unmet need. PDCs are considered one of the most promising approaches in the broader therapeutics category. The global peptide market, which includes insulin and other hormones, is expected to surpass $75 billion by 2028, according to Kuick Research, which identifies Theratechnologies, Amgen, Eli Lilly, Galena Biopharmaceuticals, Merck, Roche, Bicycle Therapeutics, and PeptiDream as among the key players in the PDC field.1
Much of the market’s promise reflects the apparent advantages of PDCs over ADCs, the latter of which have become a popular drug class for treating solid tumors and hematologic malignancies. As with most cytotoxic drugs, the emergence of resistance mechanisms limits ADCs’ clinical benefit and their duration of objective response.2 Moreover, ADCs are large, bulky biologics with a relatively long half-life that can range from as little as 16 hours to as long as 12 days, raising the risk of off-target effects including neutropenia, neuropathy, and hepatotoxicity.3,4 Such severe side effects can limit patients’ ability or willingness to adhere to the treatment regimen. Additionally, the slow internalization of most ADCs into the target cell (6 to 14 hours5) indicates that they can remain on the cell surface for an extended period, reducing the amount of drug that reaches the intracellular target and thus requiring conjugation with highly toxic drugs.6
PDCs, by contrast, are small, agile chemical entities, some of which have half-lives of just a few hours, enabling rapid internalization and improved tumor penetration within minutes. Those properties may allow PDCs to maintain their robust anticancer activity while significantly improving upon the safety profile of ADCs and may enable conjugation with different payloads, a potentially significant advantage.7,8 Two additional promising aspects of PDCs are their apparent ability to bypass drug resistance mechanisms and enhance immune checkpoint inhibition.8 PDCs are also less expensive and faster to advance from preclinical to clinical development compared to monoclonal antibodies and offer improved tumor penetration as well as greater flexibility in terms of ease of payload-switching.9
SORT1: Matching The Right Compound To The Right Target
One of the most promising targets for PDC therapy is sortilin (SORT1), a transmembrane glycoprotein of the vascular protein sorting 10 protein (Vsp10p) family. Its normal function is to act as a cellular shuttle system to transport proteins across the cell membrane and to rapidly internalize them via the endosomal/lysosomal pathway. SORT1 functions very differently from cell surface receptors such as HER2, the most common ADC target, and is highly expressed in many solid tumors, including ovarian, endometrial, and breast cancers.10-12 Although it is not a well-established cancer biomarker, a growing body of evidence supports its potential suitability as a target for anticancer therapies. In addition to its transmembrane shuttle function, SORT1 is associated with tumorigenesis, resistance, progression, advanced disease, poor prognosis, and reduced survival outcomes in cancer. Overall, there is greater expression of SORT1 in cancer cells compared to normal cells, making it an attractive research target.
SORT1-targeting PDC therapy harnesses the natural internalization function of SORT1 to promote rapid internalization and delivery of a cytotoxic payload directly into cancer cells, while limiting ligand degradation in the circulation as well as off-target toxicity.13 The SORT1 receptor rapidly internalizes its natural ligands (e.g., progranulin, neurotensin),14 expediting peptide uptake within cancer cells.15 Preclinical studies suggest targeting SORT1 with a PDC results in improved tolerability as compared to the safety profile of free cytotoxic agents or ADC therapy, as evidenced by a lack of neutropenia after multiple administrations, as well as by minimal cytotoxic payload concentrations in the plasma.16
The below figure illustrates the mechanism of sudocetaxel zendusortide (TH1902), the first PDC emerging from Theratechnologies’ SORT1+ Technology™ platform.
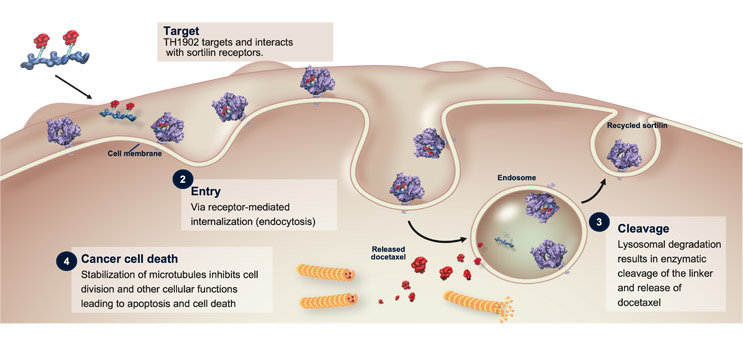
Figure 1: Sudocetaxel zendusortide (TH1902) mechanism of action17-19
The product of roughly 25 years of research experience, sudocetaxel zendusortide incorporates a SORT1-targeting peptide, TH19P01, which can be conjugated to a variety of anticancer agents with a consistent number of payload molecules. Sudocetaxel zendusortide is conjugated in a 2:1 ratio with docetaxel, a well-documented agent. In preclinical studies, TH1902 demonstrated multiple anticancer activities, including cytotoxicity, inhibition of cancer stem cells and vasculogenic mimicry, induction of apoptosis, cell cycle arrest, and immune cell infiltration. As a PDC, sudocetaxel zendusortide widens the docetaxel therapeutic window, using a smaller dose to achieve greater efficacy and less toxicity than that observed with docetaxel monotherapy. Its cleavable linker potentiates rapid release of docetaxel inside the cancer cells while limiting its release in the circulation to minimize off-target effects.20-23 Sudocetaxel zendusortide, with its versatile “conjugability,” thus has potential applications in multiple tumor types and may prove useful in other disease areas beyond oncology.
Future Directions
On the heels of the success of ADCs, PDCs may yield advances in cytotoxics and beyond, alone or in combination with targeted therapies, including checkpoint inhibitors and other anticancer treatment modalities. While early PDC trials have yielded favorable results, proven benefits in ongoing clinical trials may usher in a new wave of research and development programs with great potential. In the meantime, these next-generation conjugates and their wider platforms are spurring exploration of potential partnerships between biotechnology and pharmaceutical companies both large and small, with some companies basing their entire pipeline models on such collaborations. Those possibilities have made PDCs a category to watch.
References
- Kuick Research. Global Peptide Therapeutics Market and Clinical Insight 2028; 2023. https://www.kuickresearch.com/ccformF.php?t=1650627251
- Biopharma PEG. Overview of ADC-Based Combination Therapies; 2023. https://www.biochempeg.com/article/330.html
- Hinrichs MJM, Dixit R. Antibody Drug Conjugates: Nonclinical Safety Concerns. AAPS J. 2015;17(5):1055-1064.
- Mckertish CM, Kayser V. Advances and Limitations of Antibody Drug Conjugates for Cancer. Biomedicines. 2021;9:872.
- Maass KF, Kulkarni C, Betts AM, Wittrup KD. Determination of Cellular Processing Rates for a Trastuzumab-Maytansinoid Antibody-Drug Conjugate (ADC) Highlights Key Parameters for ADC Design. AAPS J. 2016;18(3):635-646.
- Nejadmoghaddam MR, Minai-Tehrani A, Ghahremanzadeh R, Mahmoudi M, Dinarvand R, Zarnani AH. Antibody-Drug Conjugates: Possibilities and Challenges. Avicenna J Med Biotech. 2019;11(1): 3-23.
- Wu M, Huang W, Yang N, Liu Y. Learn from Antibody-Drug Conjugates: Consideration in the Future Construction of Peptide-Drug Conjugates for Cancer Therapy. Exp Hematol Oncol. 2022;11:93.
- Chavda VP, Solanki HK, Davidson M, Apostolopoulos V, Bojarska J. Peptide-Drug Conjugates: A New Hope for Cancer Management. Molecules. 2022;27:7232.
- Alas M, Saghaeidehkordi A, Kaur K. Peptide-Drug Conjugates with Different Linkers for Cancer Therapy. J Med Chem. 2021;64(1):216-232.
- Ghaemimanesh F, Ahmadian G, Talebi S, et al. The Effect of Sortilin Silencing on Ovarian Carcinoma Cells. Avicenna J. Med. Biotechnol. 2014;6:169–177.
- Hemmati S, Zarnani AH, Mahmoudi AR, et al. Ectopic Expression of Sortilin 1 (NTR-3) in Patients with Ovarian Carcinoma. Avicenna J. Med. Biotechnol. 2009;1:125–131.
- Berger K, Rhost S, Rafnsdóttir S, et al. Tumor Co-Expression of Progranulin and Sortilin as a Prognostic Biomarker in Breast Cancer. BMC Cancer. 2021;21:185.
- Demeule M, Currie JC, Larocque A, et al. Increasing Penetration of Anticancer Drugs through Sortilin Receptor-Mediated Cancer Therapy: A New Targeted and Personalized Approach in the Treatment of Ovarian Cancer. Cancer Res. 2017;77(13 suppl): 5146.
- Hu F, Padukkavidana T, Vægter CB, et al. Sortilin-Mediated Endocytosis Determines Levels of the Frontotemporal Dementia Protein, Progranulin. Neuron. 2010;68:654-667.
- Regina A, Demeule M, Tripathy S, et al. ANG4043, a Novel Brain-Penetrant Peptide-mAb Conjugate, is Efficacious against HER2-Positive Intracranial Tumors in Mice. Mol Cancer Ther. 2015;14:129-140.
- Marsolais C, Charfi C, Demeule M, et al. A Novel Sortilin-Targeted Docetaxel Peptide Conjugate (TH1902), for the Treatment of Sortilin-positive (SORT1+) Triple-Negative Breast Cancer. Cancer Res. 2020;80(16_Supplement):2910.
- Demeule M, Currie JC, Larocque A, et al. Sortilin Receptor-Mediated Novel Cancer Therapy: A Targeted Approach to Inhibit Vasculogenic Mimicry in Ovarian and Breast Cancers. Poster 4335 presented at Annual Meeting of the American Association for Cancer Research; June 22-24, 2020: virtual.
- Makawita S, Meric-Bernstam F. Antibody-Drug Conjugates: Patient and Treatment Selection. Am Soc Clin Oncol Educ Book. 2020;40:1-10.
- Taxotere (docetaxel) Prescribing Information. Sanofi-Aventis US LLC; 2023.
- Annabi B, Demeule M, Currie JC, et al. TH1901, a Novel Curcumin-Peptide Conjugate for the Treatment of Sortilin-Positive (SORT1+) Cancer. Poster #4386 presented at Annual Meeting of the American Association for Cancer Research; June 22-24, 2020: virtual.
- Hoppenz P, Els-Heindl S, Beck-Sickinger AG. Peptide-Drug Conjugates and their Targets in Advanced Cancer Therapies. Front Chem. 2020;8:571.
- Currie JC, Charfi C, Demeule M, et al. A Novel Sortilin-Targeted Docetaxel Peptide Conjugate (TH1902), for the Treatment of Sortilin-Positive (SORT1+) Triple-Negative Breast Cancer. Poster #4472 presented at Annual Meeting of the American Association for Cancer Research; June 22-24, 2020: virtual.
- Zhang E, Zing R, Liu S, Li P. Current Advances in Development of New Docetaxel Formulations. Expert Opin Drug Deliv. 2019;16:301-31.
About The Authors:
 Christian Marsolais, Ph.D., is senior vice president and chief medical officer at Theratechnologies. He has more than 30 years of experience working in drug research, development, and commercialization. Prior to joining Theratechnologies in 2007, he was part of the global oncology team at Pfizer. He has also held positions at Sandoz (now Novartis) and Biochem. Marsolais was instrumental to Theratechnologies’ acquisition of Katana Biopharma, Inc., which discovered the SORT1 Technology platform and led to the development of sudocetaxel zendusortide. He holds a Ph.D. in biochemistry from the Université de Montréal and has also been instrumental in Theratechnologies’ HIV franchise.
Christian Marsolais, Ph.D., is senior vice president and chief medical officer at Theratechnologies. He has more than 30 years of experience working in drug research, development, and commercialization. Prior to joining Theratechnologies in 2007, he was part of the global oncology team at Pfizer. He has also held positions at Sandoz (now Novartis) and Biochem. Marsolais was instrumental to Theratechnologies’ acquisition of Katana Biopharma, Inc., which discovered the SORT1 Technology platform and led to the development of sudocetaxel zendusortide. He holds a Ph.D. in biochemistry from the Université de Montréal and has also been instrumental in Theratechnologies’ HIV franchise.
 Michel Demeule, Ph.D., is senior director of oncology research at Theratechnologies. He is a researcher with more than 30 years of experience in academia and the pharmaceutical industry. His discoveries led to the formation of two oncology companies: Angiochem and Katana Biopharma, Inc. Theratechnologies acquired the latter company, where Demeule developed the SORT1 Technology platform. He is a co-inventor of 40 patents, a co-author of 69 scientific peer-reviewed papers, and three book chapters. He has worked on all aspects of drug transport and brain delivery of molecules, including small molecules, peptides, monoclonal antibodies, and antibody–drug conjugates in oncology and other diseases of the central nervous system. Demeule completed his Ph.D. in physiology at the Université de Montréal.
Michel Demeule, Ph.D., is senior director of oncology research at Theratechnologies. He is a researcher with more than 30 years of experience in academia and the pharmaceutical industry. His discoveries led to the formation of two oncology companies: Angiochem and Katana Biopharma, Inc. Theratechnologies acquired the latter company, where Demeule developed the SORT1 Technology platform. He is a co-inventor of 40 patents, a co-author of 69 scientific peer-reviewed papers, and three book chapters. He has worked on all aspects of drug transport and brain delivery of molecules, including small molecules, peptides, monoclonal antibodies, and antibody–drug conjugates in oncology and other diseases of the central nervous system. Demeule completed his Ph.D. in physiology at the Université de Montréal.
