The 5-Layer Fix For AI Failure In Pharmaceutical Manufacturing
By Srihari Rangarajan, Nathan Silverman, Adam Cooper, and Ryan Hill

Pharmaceutical manufacturers stand at a critical inflection point, where the promise of artificial intelligence (AI) meets the harsh reality of data chaos. As companies in the industry ramp up their investments in AI — projected to surge over sixfold by 20301 — their aspirations often collide with a staggering statistic: 95% of AI pilots fail to deliver measurable business value.2
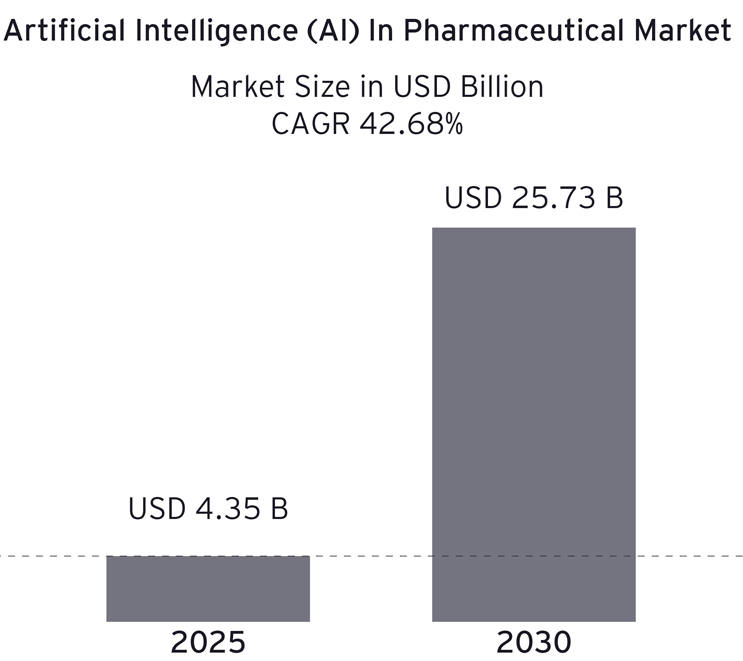
Source: Mordor Intelligence
This concerning trend doesn’t merely reflect technological shortcomings; it underscores a deeper issue rooted in the very data that fuels these initiatives. Below, we explore why traditional data architectures hinder AI success and present a proven “AI by design” approach — a five-layer data mesh strategy designed to transform chaotic pharmaceutical manufacturing data into AI-ready information without disrupting existing operations.
The Sobering Reality: Why AI Investments Fail
Manufacturing executives are all too familiar with the pattern: a promising AI pilot launches amid great expectations and hype, backed by talented teams, advanced technology, and well-defined use cases. Yet, six months later, it often ends up quietly shelved. Postmortem analysis consistently points to a single culprit: AI that performed flawlessly in the lab faltered on the factory floor because of fundamentally flawed production data.
The numbers tell the story:
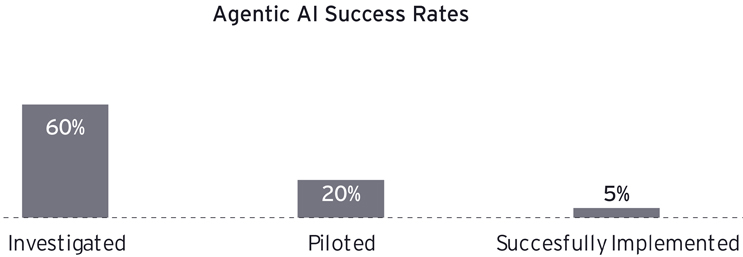
- Only 5% of enterprise agentic AI pilots achieve rapid value acceleration.3 This stark statistic highlights a pervasive challenge within the industry: Most AI initiatives fail to translate into tangible financial benefits. The low success rate underscores the critical need for organizations to reassess their approach to AI implementation, particularly in how they manage and prepare their data.
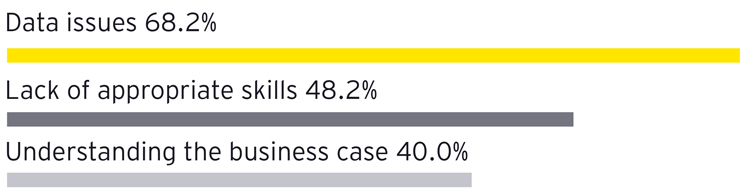
- Data issues are the biggest challenge facing AI in manufacturing.4 Many manufacturing leaders identify data quality and accessibility as the primary obstacles to successful AI integration. Poor data governance, inconsistent data formats, and a lack of real-time data availability hinder AI systems’ ability to deliver accurate insights and drive operational efficiencies.
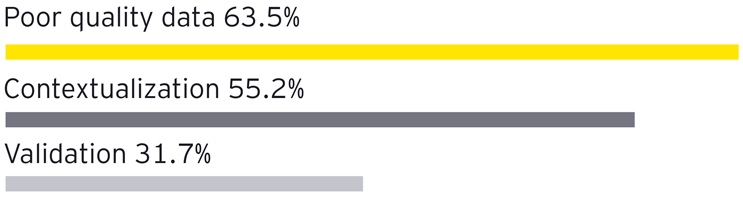
- Data quality and contextualization are the top challenges with AI data.5 High-quality, context-rich data is essential for AI systems to function effectively. Yet many organizations struggle with fragmented data sources and inadequate data management practices, leading to unreliable inputs for AI algorithms. This lack of contextualization can result in misguided AI outputs, further perpetuating the cycle of failure in AI projects.
This “pilot purgatory” phenomenon is particularly pronounced in pharmaceutical manufacturing, where complex regulatory environments, risk-averse cultures, and sprawling data ecosystems encompass R&D, clinical trials, manufacturing, and supply chain operations. The issue lies not in the sophistication of AI but in the readiness of the data it relies upon.
Understanding The Root Causes: The Seven Fatal Flaws
To grasp why AI initiatives often falter, it is essential to identify the underlying flaws in manufacturing data systems. These flaws create barriers that prevent AI from delivering its promised value. Below are seven fatal flaws that plague pharmaceutical manufacturing data and hinder successful AI implementation:
- The silo trap: Quality data can’t be contextualized against maintenance history, maintenance history can’t be related to production parameters, and production parameters aren’t connected to quality data. Defect root cause analysis often requires tedious manual merging of spreadsheet exports from disparate systems, transforming a 4-minute task into a 4-hour ordeal. Within that time, the factory has produced another thousand potentially defective units.
- The time warp problem: Discrepancies in timekeeping across machines lead to chaos. For example, Machine A uses plant local time, Machine B uses UTC, and Machine C’s clock drifts 3 minutes per month without anyone noticing. When AI analyzes data, it may see effects occurring before their causes, akin to predicting the future while sifting through scrambled historical data.
- The Tower of Babel effect: Different systems use varying terminologies for the same metrics. For instance, one system calls a metric “Product_Quality_Check,” another labels it “QC_Result,” and a third system uses “InspectionOutcome.” As a result, AI can’t make sense of the data. Models trained in one plant often fail in another because of inconsistent vocabulary — not differing processes.
- The batch bottleneck: Equipment generates data every millisecond, yet systems report only hourly summaries. When equipment fails at 2:15 p.m., AI learns about it too late — at 3:00 p.m., when hourly batch processes run. This delay turns predictive maintenance into costly reactive measures.
- The black box phenomenon: A lack of transparency in data lineage leads to distrust in AI recommendations. When operators cannot trace the origins of data or how the information has subsequently transformed, they are less likely to rely on AI insights. And when auditors and regulators ask for data lineage, the company can’t provide it.
- The ownership vacuum: Data quality suffers from a lack of ownership. IT teams manage servers, while operational teams oversee machines, but no one is accountable for data quality, resulting in persistent issues. Problems persist because fixing them isn’t anyone’s actual job.
- The skills chasm: A disconnect exists between engineers who understand manufacturing processes and data scientists who grasp AI. This gap results in technically correct but operationally useless AI recommendations. For example, AI generates a recommendation to “adjust pressure” but doesn’t specify which pressure, when to adjust it, or why it matters.
The Five-Layer Data Mesh Solution In Pharmaceutical Manufacturing
To overcome these challenges, pharmaceutical manufacturers can adopt a five-layer data mesh strategy that transforms chaotic data into AI-ready information without disrupting existing operations. This approach treats data as a product and fosters domain-driven ownership and federated governance.
Layer 1: Edge intelligence (the foundation)
Instead of collecting uncontextualized data, organizations should tag data points with relevant context at the moment of creation. This principle emphasizes decentralized data ownership, ensuring that domain experts (manufacturing operators) enrich data at its source.
Implementation strategy:
- Deploy small, cost-efficient edge gateways machine by machine.
- Include machine ID, batch number, process phase, and calibrated time stamp at data collection.
- Use Network Time Protocol (NTP) for time synchronization, which is available in most networks.
Layer 2: Unified name space (single source of truth)
When an organization establishes a unified name space, every signal has a standardized name and location. This eliminates the need to sift through multiple systems for specific data elements.
Implementation strategy:
- Publish data to organized topics: plant, area, line, machine, and measurement.
- Define naming conventions for production lines and begin publishing.
- Create self-service data discovery through standardized interfaces.
Layer 3: Common data models (universal language)
Standardizing definitions for key data elements fosters consistency across all plants and systems, creating a framework for interoperability.
Implementation strategy:
- Develop schema definitions for five to 10 core objects in pilot use cases.
- Maintain a simple registry for storing and versioning schemas.
- Validate all data against registered schemas before acceptance.
Layer 4: Federated governance (standardization and tracking without central control)
This layer emphasizes the need for comprehensive tracking of data, regardless of its location, while allowing for local implementation of standards. In the pharmaceutical context, federated governance is particularly crucial because of the industry’s stringent regulatory requirements and the need for compliance with good manufacturing practices (GxP).
By adopting federated governance, pharmaceutical manufacturers can maintain data quality and compliance across all operations, fostering a culture of accountability and continuous improvement.
Implementation strategy:
- Establish central governance bodies that set organization-wide compliance standards, which local teams then adapt to their specific processes and regional requirements.
- Maintain audit trails to satisfy regulatory requirements while enabling innovation and flexibility in operations.
- Implement domain-specific risk controls aligned with manufacturing criticality levels so that each site can address its unique challenges while adhering to overarching governance principles.
Layer 5: Continuous learning (getting smarter over time)
To keep AI effective, organizations must capture feedback on AI recommendations and outcomes, allowing for continuous improvement.
Implementation strategy:
- Deploy feedback mechanisms (e.g., thumbs up/down buttons) for operators to evaluate AI recommendations.
- Add text fields for operator notes and context.
- Implement basic model versioning and retraining workflows.
Conclusion
The technology exists, the business case is proven, and a framework for success is available. Manufacturing leaders who address their data foundations today using data mesh principles will position themselves to succeed with AI tomorrow. Those who delay this will continue to face costly pilot failures while competitors advance.
Success hinges on focused execution rather than sweeping transformations. Identify your most pressing production challenge, rectify the data feeding it using these five layers, and then apply AI. Companies that adopt this approach can expect returns on AI investments of five to 10 times, while others risk wasting millions on failed projects.
The choice is clear: Build the data foundation that enables AI success — or continue investing in sophisticated algorithms that falter on chaotic data. The AI readiness gap represents both the industry’s greatest challenge and its most significant opportunity for competitive differentiation.
References
- “Artificial Intelligence (AI) In Pharmaceutical Market Size and Share,” Mordor Intelligence, June 2025, https://www.mordorintelligence.com/industry-reports/artificial-intelligence-in-pharmaceutical-market.
- “The GenAI Divide: State of AI in Business 2025,” MIT Project NANDA, July 2025, https://mlq.ai/media/quarterly_decks/v0.1_State_of_AI_in_Business_2025_Report.pdf.
- Ibid.
- Brousell, David R., “Survey: Manufacturers See AI as a ‘Game-Changer’ as They Ramp Up Investments,” Manufacturing Leadership Council website, August 1, 2024, https://manufacturingleadershipcouncil.com/manufacturers-see-ai-as-a-game-changer-as-they-ramp-up-investments-36926/?stream=ml-journal.
- Ibid.
About The Authors:
 Srihari Rangarajan is the EY Americas Life Sciences Supply Chain Leader. With over 20 years of experience, he helps pharmaceutical, medical device, and consumer product companies design innovative, end-to-end supply chain solutions. Rangarajan advises clients at the intersection of business and technology, leveraging AI, machine learning, IoT, intelligent automation, and digital twins to drive transformation. His work focuses on planning, sourcing, manufacturing, and logistics to optimize costs, improve efficiencies, and accelerate time-to-market. Rangarajan has partnered with life sciences organizations to deliver higher-quality medicines and devices faster to patients while enhancing enterprise value through digitally driven strategies.
Srihari Rangarajan is the EY Americas Life Sciences Supply Chain Leader. With over 20 years of experience, he helps pharmaceutical, medical device, and consumer product companies design innovative, end-to-end supply chain solutions. Rangarajan advises clients at the intersection of business and technology, leveraging AI, machine learning, IoT, intelligent automation, and digital twins to drive transformation. His work focuses on planning, sourcing, manufacturing, and logistics to optimize costs, improve efficiencies, and accelerate time-to-market. Rangarajan has partnered with life sciences organizations to deliver higher-quality medicines and devices faster to patients while enhancing enterprise value through digitally driven strategies.
 Nathan Silverman is a manager in EY’s Supply Chain and Operations Practice. He specializes in helping life sciences, medtech, and consumer companies transform their business operations through a people-first approach that accelerates ROI, streamlines processes, embeds AI, and enhances customer experience. Silverman leads a portfolio of end-to-end supply chain and manufacturing transformations for clients, advising executive leaders and directing cross-functional teams across digital, operational, and organizational change. He has partnered with life sciences clients to accelerate vaccine launches, strengthen supply resiliency, and improve access to life-saving medicines worldwide.
Nathan Silverman is a manager in EY’s Supply Chain and Operations Practice. He specializes in helping life sciences, medtech, and consumer companies transform their business operations through a people-first approach that accelerates ROI, streamlines processes, embeds AI, and enhances customer experience. Silverman leads a portfolio of end-to-end supply chain and manufacturing transformations for clients, advising executive leaders and directing cross-functional teams across digital, operational, and organizational change. He has partnered with life sciences clients to accelerate vaccine launches, strengthen supply resiliency, and improve access to life-saving medicines worldwide.
 Adam Cooper is a principal in EY’s Supply Chain and Operations Practice and leads the US Manufacturing Transformation Practice. With more than 25 years of experience, he helps organizations reimagine manufacturing through digital operations, partner ecosystems, and workforce transformation. Cooper specializes in leveraging operational technologies to empower connected industrial workers, enhance analytics, and optimize production processes. His expertise spans automation, MES, asset management, sustainability, and operational excellence. He serves clients across Life Sciences, Chemicals, Consumer Goods, Utilities, and Industrial Products sectors, driving strategies that integrate technology, efficiency, and responsible practices to deliver measurable business value.
Adam Cooper is a principal in EY’s Supply Chain and Operations Practice and leads the US Manufacturing Transformation Practice. With more than 25 years of experience, he helps organizations reimagine manufacturing through digital operations, partner ecosystems, and workforce transformation. Cooper specializes in leveraging operational technologies to empower connected industrial workers, enhance analytics, and optimize production processes. His expertise spans automation, MES, asset management, sustainability, and operational excellence. He serves clients across Life Sciences, Chemicals, Consumer Goods, Utilities, and Industrial Products sectors, driving strategies that integrate technology, efficiency, and responsible practices to deliver measurable business value.
 Ryan Hill is a manufacturing data and architecture modernization capability leader at EY with 25 years of experience in IT/OT consulting and the manufacturing industry. He specializes in evaluating and implementing critical industrial systems, including MES, SCADA, Lean drivers, and advanced scheduling solutions. Hill’s expertise in standardizing data collection, modeling, and reporting has driven significant advancements in manufacturing intelligence and analytics, improving operational efficiency and productivity. His vision encompasses automation, generative AI, predictive maintenance, and smart factory technologies. He combines continuous improvement methodologies with effective change management to deliver innovative, sustainable solutions shaping the future of manufacturing.
Ryan Hill is a manufacturing data and architecture modernization capability leader at EY with 25 years of experience in IT/OT consulting and the manufacturing industry. He specializes in evaluating and implementing critical industrial systems, including MES, SCADA, Lean drivers, and advanced scheduling solutions. Hill’s expertise in standardizing data collection, modeling, and reporting has driven significant advancements in manufacturing intelligence and analytics, improving operational efficiency and productivity. His vision encompasses automation, generative AI, predictive maintenance, and smart factory technologies. He combines continuous improvement methodologies with effective change management to deliver innovative, sustainable solutions shaping the future of manufacturing.
