The Upper Nasal Space: Drug Delivery's Next Frontier
By John Hoekman, Ph.D., Impel Pharmaceuticals, Inc.

Oral formulations of drugs account for up to 75% of prescriptions from healthcare providers, and on the surface it's easy to see why: they are relatively inexpensive and easy to manufacture, may be developed with controlled-release properties, are non-invasive, offer good stability, and are relatively easy to administer and convenient for the patient.1,2
However, many diseases have acute episodes that require therapies to provide a maximal benefit as quickly as possible, and oral delivery may result in slow absorption and onset of effect.3 Orally administered drugs may also result in side effects from high systemic drug levels and high variability due to interactions with food or other orally administered drugs.4,5
There's also the gut to consider. Certain disorders, including ones of the central nervous system (CNS) such as migraine and Parkinson's disease, are associated with high rates of nausea and vomiting, and often under-appreciated gastrointestinal (GI) comorbidities, that can further impair oral absorption.1,2,6,7,8,9 Indeed, disorders of gut-brain interaction (or DGBI) are now considered to be often overlooked and poorly understood.
Intravenous (IV) administration is another option for some disorders, but this route of administration has limitations: it requires suitable location, equipment, and healthcare professionals for safe and effective administration, is not suitable in situations of agitation or non-cooperative patients or those with needle phobia, and is not suitable for "at home" administration.10,11,12,13
There is a clear need for non-injected, non-oral dosage formulations as alternatives for treating many acute conditions.
Why The Upper Nasal Space?
Drugs delivered nasally have the potential to provide a more rapid onset of activity, avoid degradation in the GI tract and first-pass hepatic metabolism, are associated with lower risk of GI-related adverse effects, can be administered independent of meals (potentially by a caregiver), and provide patient confidence and ease of use.14
"Nasal drug delivery" is a broad term for such a complex feature of the human body, though, and the site of drug deposition within the nose is an under-appreciated consideration that carries far-ranging implications.
There are numerous approved nasal drug products on the market, of which nearly all use nasal atomizers to deliver drug into the lower nasal space located near the front of the nasal cavity, just behind the nostrils.15,16,17,18,19,20 The lower nasal space includes structures such as the vestibule and the nasal turbinates. The vestibule is covered with relatively impermeable squamous epithelium that is poorly suited for drug absorption, and although the inferior and middle turbinates are covered with more permeable epithelium, they are also covered with highly motile cilia and variable amounts of mucus.
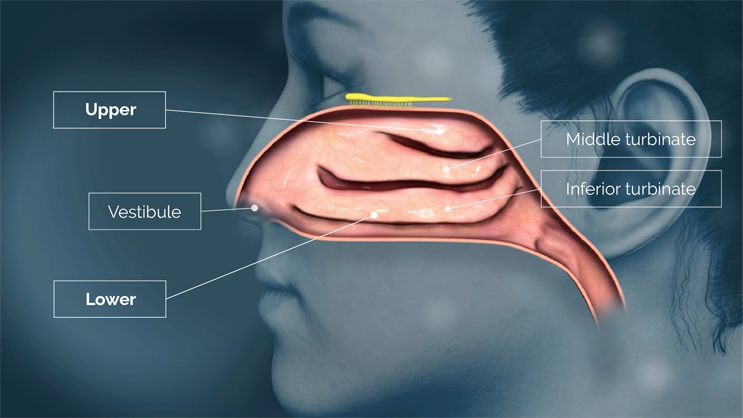
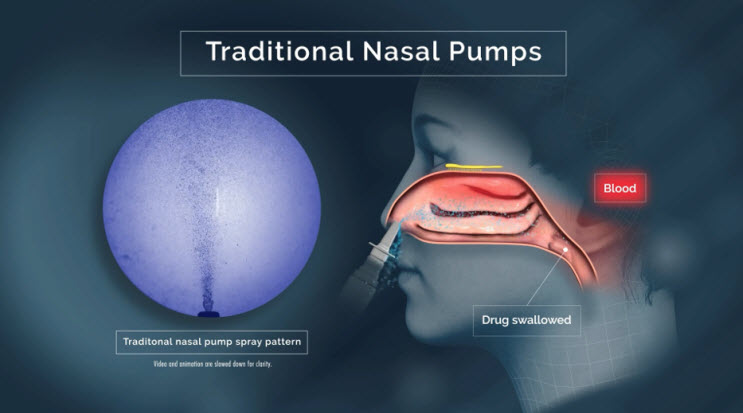
Delivering drug to the lower nasal space requires rapid absorption before gravity leads to drug dripping out of the nostril or back into the throat.15,16,17,21 Drug delivered to the lower nasal space can also get trapped in the mucus and cleared/swallowed by mucociliary clearance — an efficient protective mechanism that, unfortunately for drug developers, acts as a barrier to therapeutic delivery in the nose.22
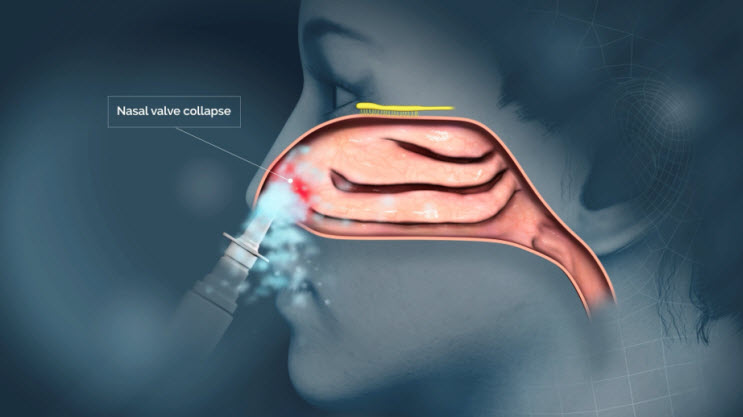
The upper nasal space, however, is an underutilized route of administration that inspired my early research and led to the development of Precision Olfactory Delivery, or POD, technology.
The upper nasal space's respiratory and olfactory epithelium areas are more permeable than the epithelium found in the lower nasal space, and the tissue architecture in the upper nasal space may render it more suitable for drug absorption.16,18,23,24,25
Within the upper nasal space is the olfactory epithelium, which has immobile cilia and therefore much slower mucociliary clearance and rich vascularization that make it more permeable than the lower space and more capable of rapid drug absorption into systemic circulation.20
Traditional sprays that deploy a "cloud" of droplets (or dry particles) only deliver around 5% of active drug to the upper nasal space, with only slight improvements after active, forced sniffing during administration.25
Impel Pharmaceuticals developed POD technology to utilize the rich vasculature found in the upper nasal space to achieve consistent and predictable drug delivery and improved bioavailability.
How Does POD Technology Work?
The POD technology is a family of handheld, manually actuated, propellant-powered administration devices that deliver drug specifically to the upper nasal space.
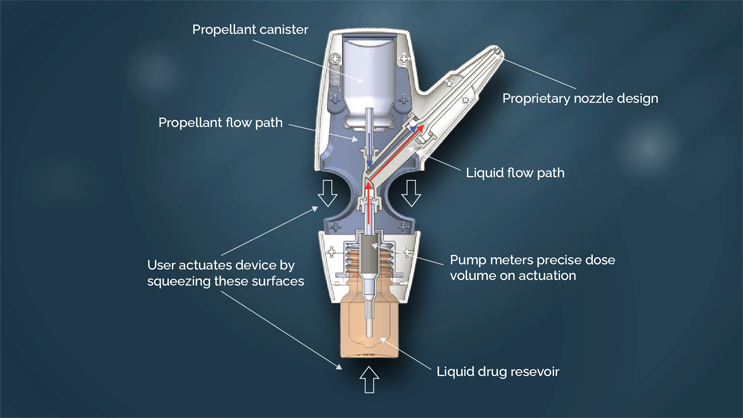
The proprietary nozzle design allows for a narrow-targeted plume of drug to effectively pass through the nasal valve to reach the upper nasal space.
The two-step action of the propellant launches the drug, and then pushes it to the farthest reaches of the upper nasal space. By maintaining the propellant and the drug (liquid or powder) separate until the time of the delivery, the POD overcomes the manufacturing challenges of maintaining dose uniformity and drug stability when drug is formulated in propellant. This allows the POD technology to deliver both liquid and dry powder formulations.
The POD technology has been shown to increase delivery of radio-label tracer to the upper nasal space versus traditional nasal pump devices. In clinical trials, this has led to improved consistency and efficiency of absorption compared to traditional nasal pumps.
The POD technology has the potential to provide rapid and consistent absorption of drug. It has been demonstrated to be suited for small molecule drugs and has the potential to be suited for large molecule drugs as well.
In the case of Impel's FDA-approved product Trudhesa (dihydroergotamine [DHE]), it delivers a plasma level similar to that found 30 minutes after IV administration, and our investigational product, INP105, achieves similar maximum level and exposure to the same active drug given intramuscularly but reaches the maximum level faster. Importantly, these consistent drug levels may result in a more reliable clinical response.27,28,29
Oral medications account for over 90% of therapies utilized to treat migraine. However, due to the several reasons mentioned above for needing an alternative to oral medications, the current American Headache Society guidelines recommend consideration of reliable, well-tolerated, non-oral routes of administration for acute therapies.3,8,15 Trudhesa was approved in September 2021 by the FDA as the first drug-device combination product to use the POD technology and the first product to successfully deliver drug to the upper nasal space and achieve therapeutic benefit for people with migraines. It avoids the undesirable high-peak plasma DHE concentration seen with IV administration while it generated similar plasma levels from 30 minutes to 48 hours.28 It also achieved a four-fold increase in maximum DHE concentration and a three-fold increase in the total amount of DHE in the blood, compared with liquid DHE nasal spray using an identical formulation but less than three-quarters of the dose.28 Patients reported that Trudhesa was easy to use in 84% of cases, and the patient survey indicated that Trudhesa allowed patients to return to normal activity faster, consistently treated their migraines, worked faster than previous treatments, and stopped migraines from coming back.28
Together, these benefits may overcome the limitations of traditional nasal delivery systems for the acute treatment of migraine and other conditions. The greater delivery consistently seen with the POD technology is paramount as it could result in a more reliable clinical response.
References
- Schiele JT, Quinzler R, Klimm HD, et al. Difficulties swallowing solid oral dosage forms in a general practice population: prevalence, causes, and relationship to dosage forms. Eur J Clin Pharmacol. 2013;69:937–948.
- Stegemann S, Gosch M, Breitkreutz J. Swallowing dysfunction and dysphagia is an unrecognized challenge for oral drug therapy. Int J Pharm. 2012;430:197–206.
- Ferrari A, Tiraferri I, Neri L, Sternieri E. Why pharmacokinetic differences among oral triptans have little clinical importance: a comment. J Headache Pain. 2011;12:5–12.
- Logrippo S, Ricci G, Sestili M, et al. Oral drug therapy in elderly with dysphagia: between a rock and a hard place! Clin Interv Aging. 2017;12:241–251.
- Tannenbaum C, Sheehan NL. Understanding and preventing drug-drug and drug-gene interactions. Expert Rev Clin Pharmacol. 2014;7:533–544.
- Lau ETL, Steadman KJ, Cichero JAY, Nissen LM. Dosage form modification and oral drug delivery in older people. Adv Drug Deliv Rev. 2018;135:75–84.
- Láinez MJ, García-Casado A, Gascón F. Optimal management of severe nausea and vomiting in migraine: improving patient outcomes. Patient Relat Outcome Meas. 2013;4:61–73.
- Aurora SK, Kori SH, Barrodale P, et al. Gastric stasis in migraine: more than just a paroxysmal abnormality during a migraine attack. Headache. 2006;46:57–63.
- Aurora SK, Papapetropoulos S, Kori SH, et al. Gastric stasis in migraineurs: etiology, characteristics, and clinical and therapeutic implications. Cephalalgia. 2013;33:408–415.
- Reisch T, Beeri S, Klein G, et al. Comparing attitudes to containment measures of patients, health care professionals and next of kin. Front Psych. 2018;9:1–8.
- Zeller SL, Citrome L. Managing agitation associated with schizophrenia and bipolar disorder in the emergency setting. West J Emerg Med. 2016;17:165–172.
- Rapoport AM, Freitag F, and Pearlman SH. Innovative delivery systems for migraine: The clinical utility of a transdermal patch for the acute treatment of migraine. CNS Drugs. 2010;24:929–940.
- Moore JC, and Miner JR. Subcutaneous delivery of sumatriptan in the treatment of migraine and primary headache. Patient Prefer Adherence. 2012;6:27–37.
- Musumeci T, Bonaccorso A, Puglisi G. Epilepsy disease and nose-to-brain delivery of polymeric nanoparticles: an overview. Pharmaceutics. 2019;11:118.
- Djupesland PG, Messina JC, and Mahmoud RA. Breath powered nasal delivery: A new route to rapid headache relief. Headache. 2013;53:72–84.
- Silberstein SD, Shrewsbury SB, and Hoekman J. Dihydroergotamine (DHE)—Then and now: A narrative review. Headache. 2020;60:40–57.
- Djupesland PG. Nasal drug delivery devices: Characteristics and performance in a clinical perspective—A review. Drug Deliv Transl Res. 2013;3:42–62.
- Martin V, Hoekman J, Aurora SK, and Shrewsbury SB. Nasal delivery of acute medications for migraine: The upper versus lower nasal space. J Clin Med. 2021;10:2468.
- Chamanza R, and Wright JA. A review of the comparative anatomy, histology, physiology and pathology of the nasal cavity of rats, mice, dogs and non-human primates. Relevance to inhalation toxicology and human health risk assessment. J Comp Pathol. 2015;153:287–314.
- Hoekman J, Brunelle A, Hite M, and Fuller C. SPECT imaging of direct nose-to-brain transfer of MAG-3 in man [Abstract]. Presented at: American Association of Pharmaceutical Scientists Annual Meeting, San Antonio, TX, November 10–14, 2013.
- Ugwoke MI, Agu RU, Verbeke N, and Kinget R. Nasal mucoadhesive drug delivery: Background, applications, trends and future perspectives. Adv Drug Deliv Rev. 2005;57:1640–1665.
- Lochhead J, Thorne R. Intranasal delivery of biologics to the central nervous system. Adv Drug Deliv Rev. 2012;64:614–628.
- Hoekman J, Ray S, Aurora SK, and Shrewsbury SB. The upper nasal space—A novel delivery route ideal for central nervous system drugs. US Neurol. 2020;16:25–31.
- Ladel S, Schlossbauer P, Flamm J, Luksch H, Mizaikoff B, and Schindowski K. Improved in vitro model for intranasal mucosal drug delivery: Primary olfactory and respiratory epithelial cells compared with the permanent nasal cell line RPMI 2650. Pharmaceutics. 2019;11:367.
- Escada PA, Lima C, and da Silva JM. The human olfactory mucosa. Eur Arch Otorhinolaryngol. 2009;266:1675–1680.
- Olafsson DR and S Gizurarson. Access to the olfactory region. Proc Control Release Bioact Mater. 2000;27:6318.
- Shrewsbury SB, Jeleva M, Satterly KH, et al. STOP 101: A phase 1, randomized, open-label, comparative bioavailability study of INP104, dihydroergotamine mesylate (DHE) administered intranasally by a I123 Precision Olfactory Delivery (POD®) device, in healthy adult subjects. Headache. 2019;59:394-409.
- Shrewsbury SB, Jeleva M, Hocevar-Trnka J, et al. STOP 301: Open-label safety and tolerability of chronic intermittent usage for 24/52 weeks of INP104 [nasal dihydroergotamine mesylate (DHE) administered by Precision Olfactory Delivery (POD®) device] in migraine headache. Presented at the International Headache Congress, Dublin, Ireland, September 5-8, 2019. Cephalalgia. 39(Suppl. 1):208-209.
- Shrewsbury SB, Hocevar-Trnka, Satterly KH, et al. The SNAP 101 Double-Blind, Placebo/Active-Controlled, Safety, Pharmacokinetic, and Pharmacodynamic Study of INP105 (Nasal Olanzapine) in Healthy Adults. J Clin Psychiatry. 2020;81(4):19m13086.
About The Author:
 John Hoekman, Ph.D., is the cofounder and chief technology and development officer of Impel Pharmaceuticals. He has spent the last 14 years investigating nasal drug delivery of therapeutics targeting the central nervous system. He cofounded Impel NeuroPharma (now Impel Pharmaceuticals) while attending graduate school at the University of Washington. He is the lead inventor of the POD technology. Hoekman has spent the last 10 years working to grow Impel from a preclinical device-focused company to a commercial-stage pharmaceutical company. He holds a BS in physics from the University of Minnesota and a Ph.D. in pharmaceutics from the University of Washington.
John Hoekman, Ph.D., is the cofounder and chief technology and development officer of Impel Pharmaceuticals. He has spent the last 14 years investigating nasal drug delivery of therapeutics targeting the central nervous system. He cofounded Impel NeuroPharma (now Impel Pharmaceuticals) while attending graduate school at the University of Washington. He is the lead inventor of the POD technology. Hoekman has spent the last 10 years working to grow Impel from a preclinical device-focused company to a commercial-stage pharmaceutical company. He holds a BS in physics from the University of Minnesota and a Ph.D. in pharmaceutics from the University of Washington.
