U.S. Sites Play Surprise Starring Role In FDA's Drug GMP Warning Letter Report
By Barbara Unger, Unger Consulting Inc.
Fiscal year (FY) 2019 was a fascinating year for drug GMP warning letters in the diversity of topics addressed, depth of focus, and trends in enforcement actions. This article presents a comprehensive summary of the drug GMP warning letters issued in FY2019, including an evaluation of trends since FY2013. The data presented for FY2019, ending Sept. 31, 2019, is based on drug GMP warning letters posted by the FDA no later than Jan. 20, 2020.
The term “compounding pharmacy,” as used here, includes outsourcing facilities and for the purpose of this analysis is considered a separate category from other drug manufacturers based on their legal foundation. These sites are located in the United States, but they are not considered with data from U.S. drug manufacturing sites in most analyses in this article. Outsourcing facilities were established by the FDA under an amendment to the Food Drug & Cosmetic Act in November of 2013. The data from these sites is included in Table 1 and Figure 1 and Table 3 (and Figure 3 in Part 2). All other tables and figures omit this market segment data. All data reflect fiscal years (with the exception of Figure 2 in Part 2, which shows the trend over time of issuance of warning letters to OTC firms in calendar years).
The narrative, tables, and figures address four broad areas, including trends between FY2013 and FY2019 in:
- Type of manufacture (API, dosage form, API and dosage form, compounding pharmacy/outsourcing facility) and country associated with the site that is the subject of the warning letters
- Time interval between the inspection and issuance of a warning letter, including data presented by compounding pharmacy sites, U.S. sites, and out of U.S. (OUS) sites.
- Particular targets of warning letters, including over-the-counter (OTC) and homeopathic drug products, drug product manufacturers, API manufacturers, and human cell therapy product (HCT/P) manufacturers
- Notable topics, including, but not limited to, warning letters identifying data integrity failures, nitrosamine contamination of angiotensin II receptor blockers (ARBs), API re-packagers, and the first warning letter citing failure to comply with requirements in the Drug Supply Chain Security Act amendments to the FD&C Act.
Five high-level conclusions can be drawn from the FY2019 warning letter data; additional conclusions are provided at the end of this article.
- Warning letters issued to firms in the U.S. far exceed the number issued to firms outside the U.S. for the first time in the eight years for which I present data (Table 1 and Figure 1).
- Warning letters to compounding pharmacies continue to decrease, following a trend that began in FY2017 after a high was reached in FY2016 (Table 1 and Figure 1).
- Warning letters that include a data integrity component continue to decrease, a trend that began in FY2017 after they reached a high in FY2016 (Figures 4 and 5).
- The intervals between inspections and warning letters continue to decrease, with the exception of warning letters issued to compounding pharmacies, where the interval increased in FY2019 (Table 3, Figure 6).
- A notably diverse group of market segments and topics are included in the FY2019 warning letter collection, including, but not limited to, failure to comply with the DSCSA, failure of repackagers to include complete information on certificates of analysis (CoAs), GMP deficiencies that led to nitrosamine contamination of ARB APIs and drug products, warning letters issued in the OTC market segment, and, finally, warning letters issued to human cell therapy producers (Figures 1, 2, and 3 in Part 2).
Overall Warning Letter Data
Table 1 and Figure 1 show that drug GMP warning letters more than doubled from FY2015 to FY2016 and continued to increase through FY2019. The increase continues but has been more gradual in the past three fiscal years. Table 1 also shows that while the FDA continues a focus on compounding pharmacies, the number of warning letters issued to these entities has decreased significantly for the past three years. The number of warning letters issued to these firms in FY2019 is approximately 34 percent of the number issued at the high point in FY2016. I doubt that this is because they have become more GMP compliant. Two factors may have contributed to this decrease : 1) many firms decided to cease the compounding of sterile injectable drugs after inspection by the FDA and 2) the FDA has taken a new and intense focus on OTC firms in the past two years. Further, regarding outsourcing facility compliance with GMPs, the FDA published draft guidance describing how outsourcing facilities might comply with GMP regulations in December 2018 and published a 51-page second draft on Jan. 22, 2020.
Note that data from compounding pharmacies/outsourcing facilities is not included, except in Table1, Figure 1, Table 3 (and Figure 3 in Part 2). All other tables and figures omit this market segment data.
Figure 2 shows that FY2019 warning letters issued to API sites are approximately half the number issued in FY2018, which was down slightly from the peak in FY2016 and 2017. The number of warning letters issued to drug product sites, however, increased substantially again this year to a total of 96 and represents 86 percent of all drug GMP warning letters issued to sites, excluding compounding pharmacies. We will address later some reasons for the increase in the number of warning letters issued to this group.
Table 1: Warning Letters Issued From FY2013 Through FY2019
|
FY * |
FY ** |
FY
|
FY
|
FY
|
FY *** |
FY **** |
|
|
TOTAL |
41 |
49 |
42 |
102 |
114 |
127 |
130 |
|
Compounding Pharmacies |
3 |
27 |
24 |
56 |
45 |
32 |
19 |
|
U.S. (non-compounders) |
13 |
4 |
3 |
11 |
20 |
22 |
68 |
|
OUS |
25 |
18 |
16 |
35 |
49 |
73 |
43 |
|
API sites |
5 |
8 |
9 |
19 |
19 |
17 |
8 |
|
Drug Product (non- compounders) |
29 |
12 |
9 |
23 |
47 |
73 |
96 |
|
API and Drug Product |
3 |
2 |
1 |
4 |
3 |
2 |
1 |
*In FY2013 one repackager is not counted as either API or drug product
**In FY2014 one warning letter regarding combination products was considered to be in the drug product category
***In FY2018 two warning letters to contract laboratories were not counted as either API or drug product
****In FY2019 one warning letter was issued to a drug distributor, two to an API repackager, one to a drug product repackager, and two to contract laboratory organizations (CLOs) that are not counted in any of the API or drug product categories
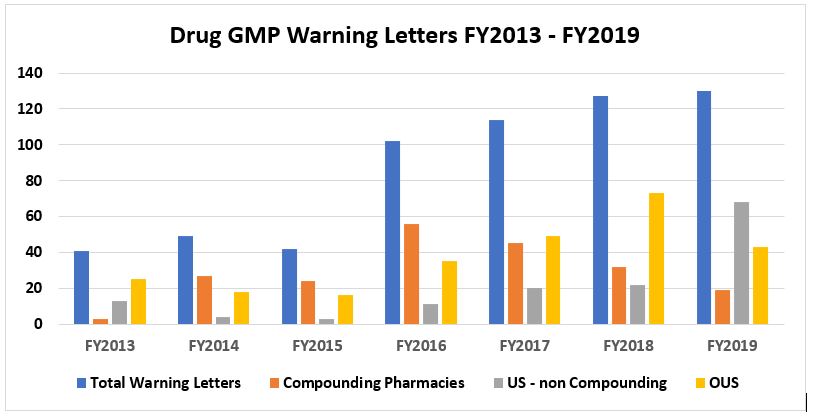
Figure 1: Warning letters issued from FY2013 through FY2019
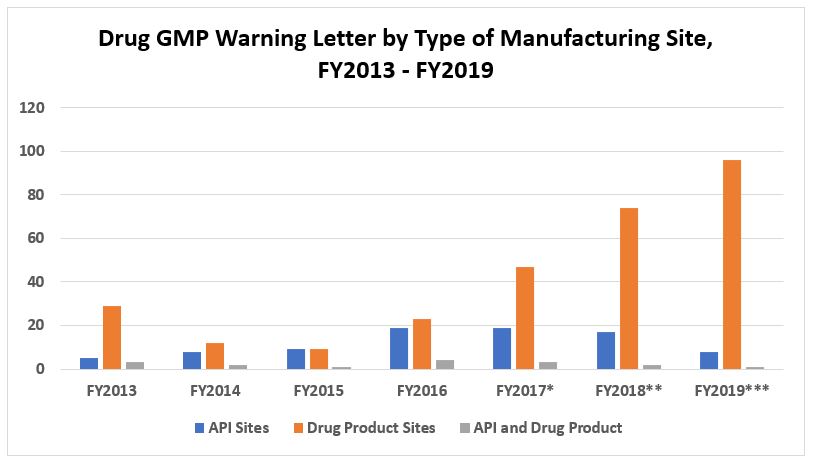
Figure 2: Drug GMP warning letters by type of manufacturing site
Table 2 presents the locations of the firms outside the U.S. that were the subject of warning letters in FY2013 through FY2019. The number of warning letters issued regarding sites in India increased slightly from FY2017 and FY2018. Firms in China, however, saw a dramatic decrease in warning letters from the previous year and the number is now more consistent with values from FY2016 and FY2017. Last year, sites that were the subject of warning letters were located in 10 countries outside the U.S., and this year that number fell to nine.
Table 2: Countries Outside the U.S. Where Firms Receiving Warning Letters Were Located
|
|
FY2013 |
FY2014 |
FY2015 |
FY2016 |
FY2017 |
FY2018 |
FY2019 |
|
Country / Geography |
|
|
|
|
|
|
|
|
India |
7 |
7 |
8 |
10 |
14 |
14 |
16 |
|
Europe |
7 |
3 |
3 |
5 |
8 |
9 |
2 |
|
China |
2 |
5 |
2 |
15 |
17 |
24 |
15 |
|
Canada |
4 |
1 |
1 |
3 |
5 |
1 |
|
|
Taiwan |
1 |
2 |
2 |
1 |
|||
|
Australia |
1 |
1 |
3 |
||||
|
New Zealand |
1 |
||||||
|
Jamaica |
1 |
||||||
|
Japan |
2 |
1 |
3 |
3 |
|||
|
Mexico |
1 |
3 |
|||||
|
Thailand |
1 |
||||||
|
Brazil |
2 |
1 |
|||||
|
Singapore |
1 |
2 |
|||||
|
South Korea |
2 |
9 |
4 |
||||
|
Dominican Republic |
1 |
||||||
|
Turkey |
1 |
||||||
|
Costa Rica |
1 |
Figure 3 shows the distribution of all drug warning letters issued in FY2019 based on geographic region or countries where the firms are located. FY2019 is notable because this is the first year that warning letters issued to firms in the U.S. exceed those issued regarding sites outside the U.S. since I began monitoring this in FY2013. These data show that significantly more than half of the warning letters in FY2019 were issued to firms in the U.S. China and India together continue to be locations for a significant number of warning letters issued OUS. This shift in location of recipients of warning letters begs the question whether the number of inspections outside the U.S. decreased and those inside the U.S. correspondingly increased in FY2019. Dr. Woodcock presented data in her testimony before Congress on Dec. 10, 2019 showing this is not the case. The FDA conducted 698 inspections in the U.S. and 966 outside the U.S. in FY2019. Dr. Woodcock’s testimony states that “FDA’s Inspections of foreign drug manufacturing facilities increased sharply after 2006 and have exceeded inspections of domestic drug facilities since 2015.” Thus, even with fewer inspections conducted in the U.S., the number of warning letters is markedly higher.
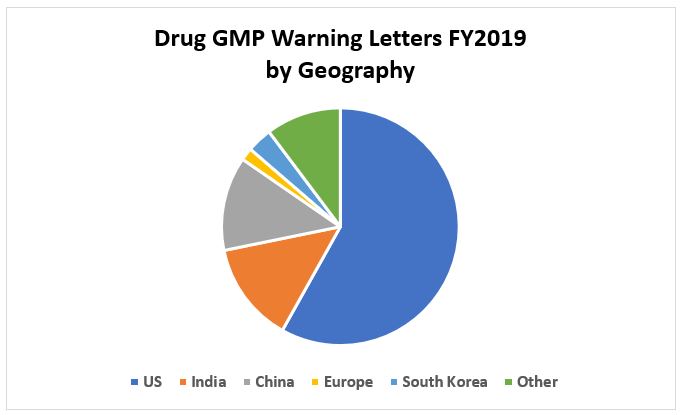
Figure 3: FY2019 warning letters by geographic location of firms
Data Integrity
Rather than writing a separate article on warning letters that identified deficiencies in data integrity, this year I’m consolidating that information here. Figure 4 shows that the percentage of all warning letters with a data integrity component continues to decrease after reaching a high of almost 80 percent in FY2016. Warning letters to sites outside the U.S. with a data integrity component also continue to decrease. Sites in the U.S., however, are up slightly over last year, perhaps due to the increased number of warning letters issued to firms here. It is also interesting to note that 38 percent (20 total) of warning letters to OTC/homeopathic manufacturers included a data integrity component. In FY2019, the percentage of warning letters issued to sites in the U.S., OUS, and overall is remarkably similar at just under 50 percent.
Figure 5 shows the geographic distribution of warning letters with data integrity deficiencies. The U.S. takes first place this year with 32, India was second with nine, and China was third with six. The remaining five warning letters are divided among other countries.
Although the absolute number of warning letters with a data integrity component issued to India and China this year seems reasonably low, it is useful to evaluate these as a percentage of the total number of warning letters issued to firms in the country. The data below result from calculation of data in Table 2 and Figure 5.
- 56 percent of the warning letters issued to firms in India included a data integrity component
- 40 percent of the warning letters issued to firms in China included a data integrity component and
- 47 percent of the warning letters issued to firms in the U.S. included a data integrity component.
The deficiencies cited in this area have not changed much since this topic was first identified more than 15 years ago. Firms continue to fail to exercise adequate controls over computer systems, fail to review all analytical raw data, fail to review audit trails, have demonstrable disagreement between electronic data and paper data that address the same event, including batch records and human machine interfaces (HMI), discard both electronic and paper based GMP data, and abort electronic analytical test runs without adequate reasons.
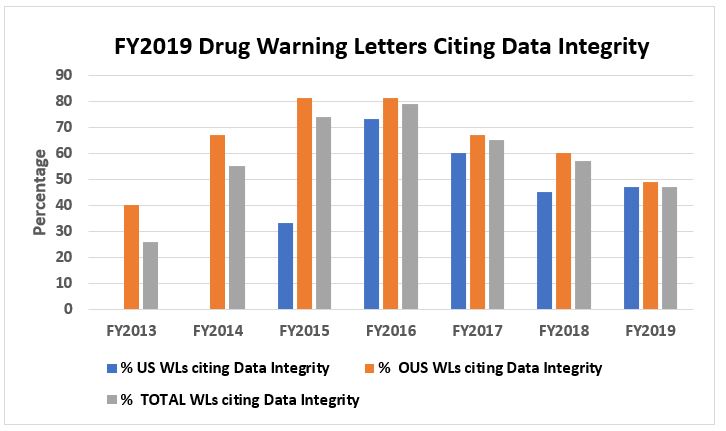
Figure 4: Warning letters with a data integrity component
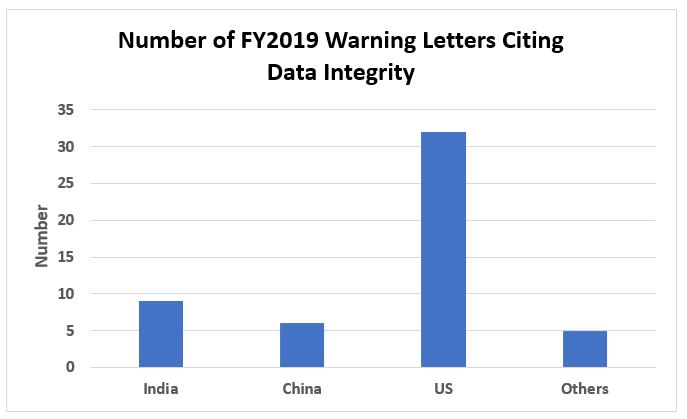
Figure 5: Number of warning letters citing data integrity by geography
Interval Between Inspections And Warning Letters
Table 3 and Figure 6 present the time interval between the FDA inspections and issuance of warning letters. These data are rounded to the nearest half month. It has been a stated goal of the FDA to substantially decrease this interval to a target of six months. The interval for issuance of warning letters for compounding pharmacies continues to be problematic, increasing this year to 15.5 months. The interval for warning letters issued to sites in the U.S. has continued to decrease from a high of 10.9 months in FY2016 to 7.2 months in FY2019. For firms outside the U.S., the interval also decreased from a high of 10.4 months in FY2015 to 6.6 months in FY2019. Taking all warning letters together, the time interval has decreased from a high of 11.9 months in FY2016 to 8.2 months in FY2019, the same interval as in FY2018.
Among the warning letters issued to sites OUS, 26 import alerts were issued in the category of failure to follow drug GMPs, distribution of unapproved new drugs, and refusal of inspection. Thus, 60 percent of the firms that received a warning letter were placed under one of these import alerts.
Table 3: Months Between Inspections and Warning Letters
|
All WLs |
Compounding Pharmacies/Outsourcing Sites |
U.S. |
OUS |
|
|
FY2013 |
8.4 |
3.7 |
10.1 |
7.8 |
|
FY2014 |
8.6 |
10.2 |
7.1 |
6.8 |
|
FY2015 |
10 |
10.4 |
4.5 |
10.4 |
|
FY2016 |
11.9 |
13.1 |
10.9 |
10.4 |
|
FY2017 |
10.1 |
12.6 |
9.6 |
8 |
|
FY2018 |
8.2 |
12.6 |
7.4 |
6.8 |
|
FY2019 |
8.2 |
15.5 |
7.2 |
6.6 |
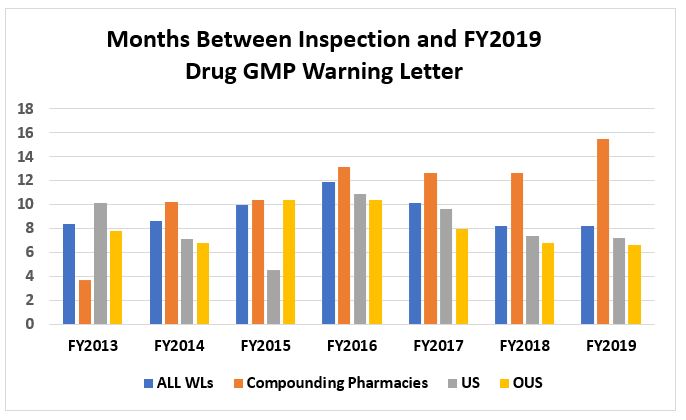
Figure 6: Months between inspections and warning letters
Conclusion
FY2019 was a fascinating year for drug GMP warning letters in the diversity of topics addressed, depth of focus, and trends in enforcement actions. The most significant news is that the number of warning letters issued to sites in the U.S. increased significantly. For the first year since I started monitoring this in FY2013, warning letters issued to firms in the U.S. constituted a majority of the drug GMP warning letters, far outpacing India and China combined.
Warning letters issued in FY2019 were issued to a diverse group of firms both inside and outside the U.S. The numbers issued to OTC firms and HCT/P firms both increased. We saw a decrease, however, in the number of warning letters issued to compounding pharmacies/outsourcing facilities that began in FY2017. Similarly, we saw a decrease in the number of warning letters that identify data integrity shortcomings, again for the third year.
Part 2 of this article will take a deeper dive into drug product manufacturers, including OTC and homeopathic drug manufacturers, human cell therapy products, nitrosamine contaminants in APIs and drug products, API repackagers, and supply chain integrity.
About The Author:
 Barbara Unger formed Unger Consulting, Inc. to provide GMP auditing and regulatory intelligence services to the pharmaceutical industry, including general GMP auditing, auditing gene and cell therapy manufacturers and suppliers, and auditing and remediation in the area of data management and data integrity. Her auditing experience includes leadership of the Amgen corporate GMP audit group for APIs and quality systems. She also developed, implemented, and maintained the GMP regulatory intelligence program for eight years at Amgen. This included surveillance, analysis, and communication of GMP related legislation, regulations, guidance, and industry compliance enforcement trends. Unger was the first chairperson of the Rx-360 Monitoring and Reporting work group that summarized and published relevant GMP and supply chain related laws, regulations, and guidance. She co-led the Rx-360 Data Integrity Working Group from 2017–2019 leading the effort to publish two manuals on how to audit and identify data integrity failures. You can contact her at bwunger123@gmail.com.
Barbara Unger formed Unger Consulting, Inc. to provide GMP auditing and regulatory intelligence services to the pharmaceutical industry, including general GMP auditing, auditing gene and cell therapy manufacturers and suppliers, and auditing and remediation in the area of data management and data integrity. Her auditing experience includes leadership of the Amgen corporate GMP audit group for APIs and quality systems. She also developed, implemented, and maintained the GMP regulatory intelligence program for eight years at Amgen. This included surveillance, analysis, and communication of GMP related legislation, regulations, guidance, and industry compliance enforcement trends. Unger was the first chairperson of the Rx-360 Monitoring and Reporting work group that summarized and published relevant GMP and supply chain related laws, regulations, and guidance. She co-led the Rx-360 Data Integrity Working Group from 2017–2019 leading the effort to publish two manuals on how to audit and identify data integrity failures. You can contact her at bwunger123@gmail.com.
