Ultraviolet Treatment Achieves Sanitary Criteria For Ultrapure Water

Aquafine's Universal Sanitary Design water treatment product
UV treatment destroys microorganisms without chemicals and their potentially harmful byproducts. The USD series is recommended for any ultrapure application with stringent quality or performance criteria, e.g. FDA, cGMP, and 3A/USDA. All 10 standard models can be configured for TOC reduction, as well as disinfection, chlorine destruction, and ozone destruction. Ranging in output from 40 to 630 GPM (9.1 to 143.2 m3/hr), the units serve most medium flow rate processes.
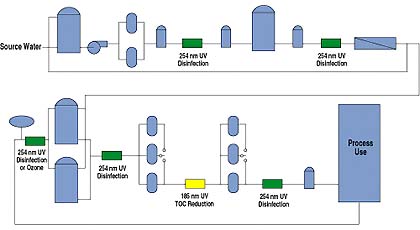
Disinfection for bio/pharmaceutical water may involve several UV units
Aquafine uses advanced manufacturing methods to create its UV treatment equipment, for example T-drill connections and orbital welding to assure longevity and purity. Sanitary inlet/outlet connections are standard on all units and sanitary ferrule endplates replace the normal bolted and threaded design. Aquafine's Aqualogic 2000T microprocessor control system provides continuous, detailed UV system feedback, including absolute real time UV intensity measurement for validation and audited production processes. The unit may be configured to monitor the system from any desktop via the 4-20mA output signal. Total access to UV intensity, temperature, lamp status, running time, and cycle count is available through a personal computer.
For more information: John Strohm, Aquafine, 25230 West Avenue Stanford, Valencia, CA, 91355. Tel: 661-257-4770. Fax: 661-257-2489.
