Using Preliminary Hazard Analysis To Determine Equipment And Instrument Requalification Frequency
By Melissa Stappen, ValSource, LLC

During the course of your career in the pharmaceutical industry, you will likely face the question, “How often should [given task or activity] be performed?” I was asked this question earlier in my own career, when I was tasked with developing an equipment qualification program. I knew the necessary steps to perform the initial qualification of equipment and instruments, and yet the nature of the equipment and instrument lifecycle was, at the time, elusive. I knew that the equipment should be periodically requalified if its consistent performance was deemed necessary to ensure the quality of products. This article explains how I developed a requalification decision-making framework based on the use of the preliminary hazard analysis (PHA), a tool used in quality risk management.
An Introduction To Equipment Qualification
Equipment qualification is a critical step in overall process validation (PV), typically referred to as Stage 2a of the PV lifecycle outlined in many regulation and guidelines, including the FDA’s 2011 Process Validation Guidance1 and Annex 15 of the EU GMPs2, among others.
A robust equipment qualification program should require that all new equipment, prior to use in production or quality control testing, pass through all stages of qualification — including design qualification (DQ), installation qualification (IQ), operational qualification (OQ), and performance qualification (PQ) — as appropriate based on the uses and risks of the equipment. The activities of each of these qualification steps should be well documented, to provide evidence that the equipment is fit for its intended purpose, and those records should be available for review by request.
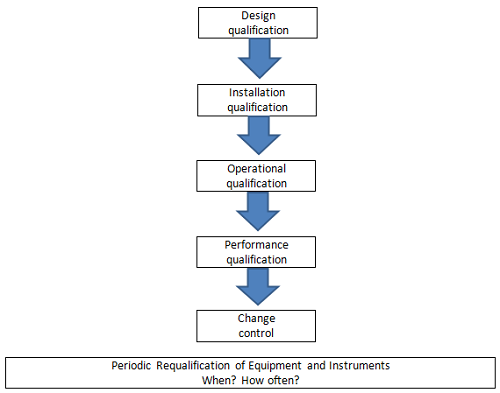
Figure 1: Stages of equipment and instrument qualification3
As illustrated in Figure 1, the equipment qualification program should speak to the periodic requalification of equipment once it has been initially qualified — and as part of implementing any changes (through a defined change control process) that may impact the qualified state of the equipment. The extent of the requalification should be based on the criticality of the equipment used in production or on the quality control testing of products and the extent of the change. A documented periodic review of pertinent data should also be performed to confirm that a process/method/system continues to consistently produce a result meeting predetermined acceptance criteria.4
ICH Q9 On Preliminary Hazard Analysis (PHA)
Returning to the personal example I shared at the beginning of this article, as I began to outline the necessary steps to perform the periodic review and requalification, I wondered, “When or at what frequency should the periodic review be performed?” I was stumped. How should I structure the program to help the user determine when to perform the review? What guideline should I follow to create this aspect of the qualification program? Where do I find the answer? After a few minutes of racking my brain, I reached for ICH Q9 (Quality Risk Management) and found my answer in Annex I — the risk management tool PHA.
ICH Q9 defines PHA as “a tool of analysis based on applying prior experience or knowledge of a hazard or failure to identify future hazards, hazardous situations and events that might cause harm, as well as to estimate their probability of occurrence for a given activity, facility, product or system.”5
The tool consists of the following components: 1) identification of the possibilities that the risk event happens, 2) qualitative evaluation of the extent of possible injury or damage to health that could result, 3) a relative ranking of the hazard using a combination of severity and likelihood of occurrence, and 4) identification of possible remedial measures.5
I realized that by building a worksheet to organize the analysis and assist with facilitation of the activities, the PHA tool could essentially address all the aforementioned questions, as well as correlate the risk priority ranking to a periodic review frequency for the subject equipment. (See Figure 2 for an example PHA worksheet.) A team consisting of equipment owners, process owners, and quality assurance could then be identified to perform the analysis. Each member should possess a strong understanding of the quality risk management process, how to use the PHA tool, scoring criteria, and key definitions.6
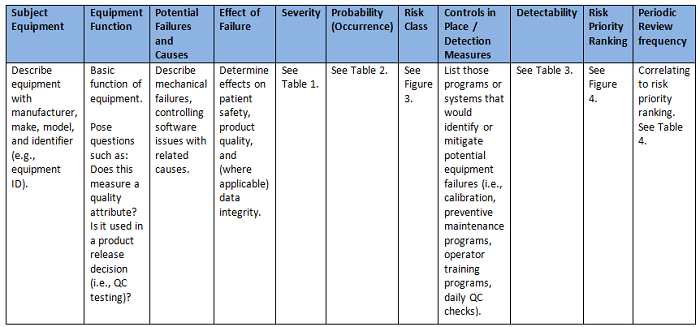
Figure 2: PHA worksheet example
The inputs to the worksheet are directed by each column and provided by a two-step triage approach using risk blocks scored against criteria as high, medium, and low rankings (see Tables 1, 2, and 3).7
Table 1: Severity Rankings
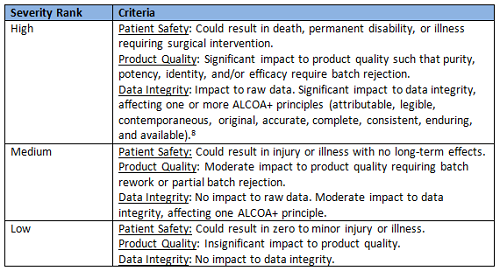
Table 2: Probability (Occurrence) Rankings

Table 3: Detectability Rankings

The preliminary risk class based on severity and probability (or occurrence) was first determined (Figure 3) and then further evaluated against detectability, resulting in a risk priority ranking (Figure 4) that correlates to the periodic review frequency for the subject equipment (Table 4).
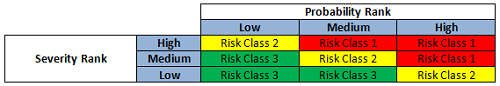
Figure 3: Risk class determination
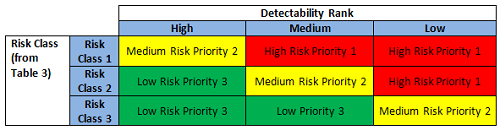
Figure 4: Risk priority ranking
Table 4: Example of Periodic Review Frequency

Once the worksheet is completed with input from team members, the periodic review frequencies should be reviewed and agreed to by the team members.
The following PHA worksheet (Figure 5) provides an example of a completed analysis activity.
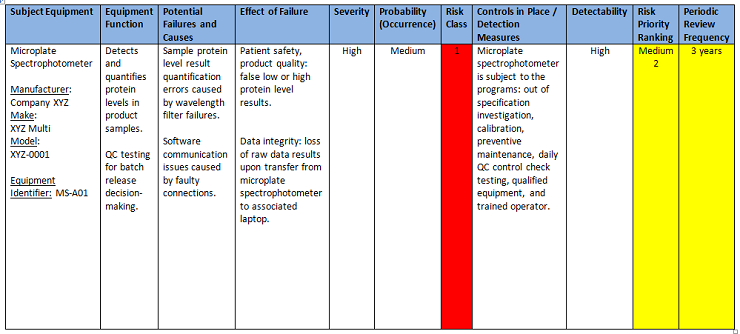
Figure 5: PHA worksheet example — completed analysis activity
My experience using the PHA tool enabled me to build a useable worksheet that allowed all members of the team to participate in the risk analysis activity. It provided each member the ability to provide subject matter expertise, voice concerns, and promote issues for further discussion. Additionally, by facilitating the team meetings, time schedules were managed and those issues that were a challenge were tabled and discussed offline. At the end of each completed worksheet, team members provided positive comments regarding the risk analysis activity, noting the efficient use of time, effective use of risk management tools, and delivery of risk priority rankings for subject equipment that correlated to a periodic review frequency.
In conclusion, using a risk management tool such as preliminary hazard analysis, a periodic review frequency can be identified and applied to qualified equipment. This will enable a timely review of the equipment by means of pertinent data such as manufacturing performance trend data, change history, and/or deviation history to confirm consistent production results meeting the predetermined acceptance criteria.
References:
- FDA. Process Validation: General Principles and Practices. January 2011.
- EU. EudraLex - Volume 4, Good Manufacturing Practice (GMP) guidelines, Annex 15: Qualification and validation. October 2015.
- Choudhary, A. Qualification of System and Equipment in Pharmaceuticals. Pharmaceutical Guidelines. December 2010.
- PDA. Technical Report No. 54-5: Quality Risk Management for the Design, Qualification, and Operation of Manufacturing Systems. May 2017.
- ICH. ICH Q9: Annex 1: Risk Management Methods and Tools. June 2005.
- PDA. Technical Report No. 54-2: Implementation of Quality Risk Management for Pharmaceutical and Biotechnology Manufacturing Operations, Annex 1. June 2013.
- GAMP 5 Guide. Compliant GxP Computerized Systems. February 2005.
- MHRA. ‘GxP’ Data Integrity Guidance and Definitions. March 2018.
About The Author:
 Melissa Stappen, consultant with ValSource, LLC, has over 20 years of experience in the pharmaceutical, medical device, biotech, and clinical/healthcare provider settings. During her career, she has provided support to quality assurance, quality control, and compliance departments as a subject matter expert relating to endotoxin and microbial contamination control. Her current role of validation consultant emphasizes risk management based programs for laboratory instrumentation, methods, and equipment. You can email her at mstappen@valsource.com or connect with her on LinkedIn.
Melissa Stappen, consultant with ValSource, LLC, has over 20 years of experience in the pharmaceutical, medical device, biotech, and clinical/healthcare provider settings. During her career, she has provided support to quality assurance, quality control, and compliance departments as a subject matter expert relating to endotoxin and microbial contamination control. Her current role of validation consultant emphasizes risk management based programs for laboratory instrumentation, methods, and equipment. You can email her at mstappen@valsource.com or connect with her on LinkedIn.
