USP Selects Six Students for 2003 Summer Internship Program

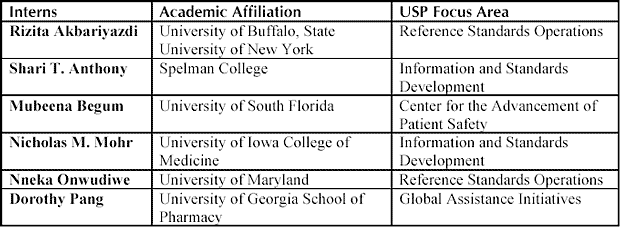
USP selected the interns based on their enhanced appreciation and understanding of drug standards setting, information development, and medication error reporting programs. In addition, USP anticipates that the interns will apply their USP summer experience to modern health practices. Each intern is awarded $8,500 stipend for the 12-week program, which begins May 19, 2003, and concludes Aug. 8, 2003.
Summer interns will contribute their classroom experience, skills, and abilities to a different USP focus area:
Global Assistance Initiatives
• Providing technical assistance in international drug quality assurance and drug information development and dissemination
Reference Standards Operations
• Developing a stability database
Center for the Advancement of Patient Safety
• Promoting medication error reporting
• Conducting data analysis and researching and developing educational programs
• Proposing standards, recommendations, and guidelines that ultimately improve the safety and quality of patient care
Information and Standards Development
• Developing formulas for compounded preparations
• Evaluating needs and data reports on pharmacy compounded preparations from the R&D Laboratory
• Identifying, evaluating, and proposing changes to monographs of the United States
Pharmacopeia and National Formulary (USP-NF)
• Developing nomenclature policies
For more information about USP's 2003 Summer Internship Program, contact Clydewyn M. Anthony at 301/816-8139, visit www.usp.org/fellows-interns, or send an e-mail to Fellows/Interns@usp.org.
