3 Ways To Deliver A Pharma 4.0 Facility

By Katie Anderson, Chief Editor, Pharmaceutical Online

“Are we moving fast enough as an industry?” asked Gunter Baumgartner in his opening remarks at the 2025 ISPE Annual Meeting & Expo. Though the pharmaceutical industry certainly has its challenges—with high implementation costs, legacy systems, cybersecurity threats, workforce shortages, skills gaps and sustainability pressures cited by Baumgartner—after learning about the latest advancements and designs at the event, the only answer to Baumgartner’s question is yes.
To get there, Baumgartner stressed the need for true digital transformation, agility, collaboration, data-driven decisions and a center around the patient.
Brendan O’Callaghan then took attendees on Sanofi’s journey to modernization, one that resonated with many as perhaps a blueprint by which they can bring their manufacturing to Pharma 4.0 level, the theme of the event. O’Callaghan explained that modernization seems daunting, but with the right plan, it doesn’t’ have to be intimidating. “Sometimes we over engineer and drive a lot of complexity into what we do,” he added. O’Callaghan supplied three ways in which Sanofi brought its sites to the modern era, which were supported throughout the event’s presentations.
Address Manufacturing Efficiency
With 39 manufacturing sites and 30+ new products projected by 2030, Sanofi needed to modernize its manufacturing to move at a rapid pace. He recommended looking at the top 10% and figuring out from there how to close the gap.
Sanofi has two AI-enabled facilities in France and Singapore. There, they are able to produce up to four vaccines or biologics simultaneously through a modular manufacturing process they call Modulus. These modular manufacturing sites enable the company to reduce changeover from months to days.
Rod Hoffman also discussed his transition to flexible modular plant installations at AstraZeneca. Not only does modular production speed up project delivery, according to Hoffman, but it also builds quality and champion’s sustainability.
Sanofi has brought in more automation and robotics. O’Callaghan discussed humanoid robots as the future of pharmaceutical manufacturing, not to replace humans but to free them up for other tasks. “These robots take on exhausting tasks to free up time for what matters,” he added.
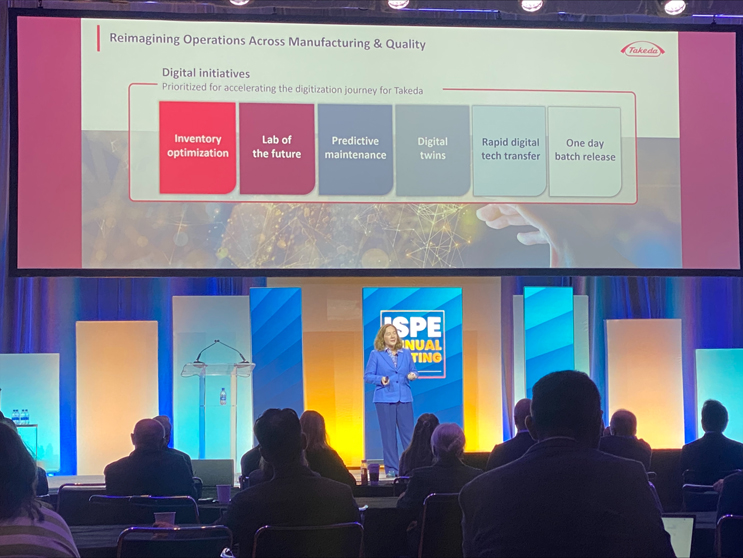
In her presentation, Elaine Shannon of Takeda echoed, “AI is reshaping our business at an unprecedented pace.” Her company has a predictive maintenance system using AI and machine learning digital agents to detect and diagnose equipment issues early. It can reduce equipment failures by 20-30%. Other digital initiatives in quality control at Takeda include: inventory optimization, lab of the future, digital twins, rapid digital technology transfer and one day batch release.
In 2022, Sanofi launched its first Digital Accelerator, which used AI, data and digital intelligence to develop products and solutions. Five of the company’s 39 sites are also “digital lighthouses,” where their fully-digitized processes serve as an example to other facilities.
Digital Excellence Cockpits at Sanofi manage daily routines to ensure consistent execution. The brand has one-click tech transfers, where digital and AI advanced technology run predictive simulations of new processes.
Invest In Your Team
One way Sanofi has ignited its team is by establishing a stronger site network for better collaboration. It has simplified, standardized and digitized its processes to set its team up for success.
O’Callaghan also introduced the idea of “accelerating a performance mindset.” The company partnered with McLaren, who has set the standard for performance with Formula 1 cars. He added that they have rethought their performance and competitive mindset, leveraging technology and analytics.
Shannon discussed how Takeda is investing in the continuous learning of their people. Each employee is allowed three hours of continuous learning per month to invest in their skill development. At Takeda, there is a large focus on enhancing digital skills, and digital learning engagement is up 20% year over year there. She added, “The digital transition is led by empowering teams.”
Think About Your Purpose
At the end of the day, all of our efforts in pharma manufacturing must serve the patient. O’Callaghan discussed how the patient is always at the center, noting that it has a program to supply its medicines at no cost to those who qualify. This year, it also unveiled a program for those with diabetes to cap their monthly medicine costs to $35.
Sanofi is also committed to the planet with a goal of net-zero greenhouse gas emissions by 2045 and a carbon neutral goal by 2030. It has also applied a sustainable by design concept.
In her presentation on digitizing quality control, Shannon noted that the evolution at Takeda always keeps the patient at the hub.
As the environmental and sustainability lead for AstraZeneca, Kevin Conkright shared his team’s efforts to reduce its carbon footprint at the company’s Mt. Vernon site. The site is near zero, with a 77.5% reduction in greenhouse gas emissions and 63% of the fleet now battery vehicles. The site also has a 23% reduction in water use, and it acquired a nearby solar field to produce renewable energy. It replaced its natural gas boilers with electric boilers to supply clean heat. It has also replaced 100,000 fixtures to reduce its energy consumption. Overall, the company has a commitment to plant 400 million trees in six countries by 2030. Conkright concluded that “change is your friend, even if you dislike it.” He encouraged attendees to think as an entrepreneur, and that time is short.
A Continuous Evolution
Regarding digitization, Baumgartner asked in his opening presentation, “Where are we on our journey?” Though the answer differs by company, one thing remains clear—digitization and modernization are necessary in pharmaceutical manufacturing. And, as Conkright noted, “the path is never-ending.” We as an industry must continue to evolve and modernize to keep up not only with demand and industry pressures, but also the resources in our environment and the skillset of the future.
