8 Ways To Drive Resilience In Oncology Drug Supply Chains
By Kerim Ozbilge, EY
Cancer continues to cast a long shadow over human health globally, standing as one of the leading causes of death and profoundly impacting millions of lives and families each year. The statistics are staggering: By 2050, we could see as many as 35 million new cases and 19 million deaths, according to the International Agency for Research on Cancer.1 In response to the rising incidence of cancer, the life sciences sector is mobilizing with unprecedented vigor, channeling substantial investments into an array of modalities and therapeutic areas, each presenting unique supply chain and business requirements and complexities. Below, we discuss the sector’s response to the growing burden of cancer and explore strategic measures that life sciences organizations should take to address a wide range of challenges.
Emerging Cancer Therapies’ Varying Challenges
The global oncology market is rapidly expanding, driven by significant investments in R&D that are leading to a robust pipeline of innovative drugs and therapies. Collaborations among biotech firms, academic institutions, and healthcare providers are accelerating the discovery and delivery of advanced cancer treatments, with the market projected to grow at a compound annual growth rate of 11.5% to 14.5%, potentially reaching over $400 billion by 2028, according to the IQVIA Institute for Human Data Science.2 While traditional chemotherapeutics remain mainstay cancer treatment options, advances in biotechnology, genomics, and personalized medicine are paving the way for targeted therapies and immunotherapies that significantly improve patient outcomes. A class of novel modalities is emerging, focused on precision medicine to tailor treatments based on an individual’s genetic profile. These include targeted therapies such as tyrosine kinase inhibitors, monoclonal antibodies, and antibody-drug conjugates, among others.
The landscape of cancer treatment is diverse, featuring a broad range of therapies that each present unique supply chain challenges, requiring specialized processes and infrastructure. From traditional chemotherapy to advanced immunotherapies and nanotechnology, the development and distribution of these therapies involve overcoming logistical, regulatory, and manufacturing hurdles that affect their availability and quality. For example, immunotherapy depends on advanced biotechnological processes and cold chain logistics, while therapies like radiopharmaceuticals and hormone therapies require specialized facilities and rigorous compliance. As the life sciences sector addresses the growing cancer burden, executives must adopt strategic actions that specifically target these distinct challenges. By doing so, they can enhance the resilience and efficiency of oncology supply chains while demonstrating their commitment to innovation and improved patient care in a complex healthcare landscape.
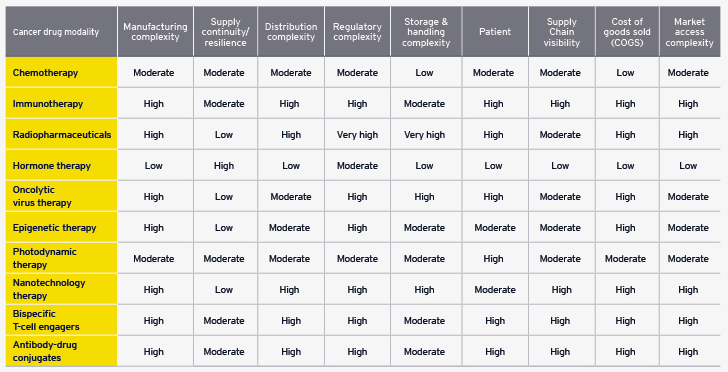
The realm of cancer treatment encompasses a wide array of therapies, each with distinct complexities and supply chain challenges that necessitate tailored processes and infrastructure.
Strengthening Cancer Treatment Supply Chains
Addressing the supply chain complexities and unique requirements associated with each cancer therapy requires life sciences organizations to adopt comprehensive and agile strategies. These organizations need a multifaceted approach to ensure that their lifesaving cancer therapies are consistently available to patients in a timely manner while preserving the therapies’ quality, improving efficiency, and reducing cost. This involves well-defined supply chain network strategies, as well as the integration of advanced manufacturing technologies, implementation of rigorous quality control measures, and development of robust logistics and supply chain management systems.
By focusing on the following key priorities, life sciences organizations can establish highly reliable and resilient supply chains along with their ecosystem partners — strengthening their ability to deliver high-quality cancer therapies to patients worldwide efficiently and effectively.
1. Design a Future-Ready Supply Chain Strategy
To build an effective supply chain, life sciences organizations should adopt a holistic approach that considers current needs and future scalability. The specificity of each cancer therapy modality necessitates adaptable approaches to drive the consistent availability of these therapies. This involves segmenting the supply chain based on diverse product portfolios and creating tailored operating models.
Organizations must prioritize a pragmatic road map to implement scalable capabilities across business functions. Many have diverse product portfolios that include both R&D and commercial products. Whether an organization is a small biotech or a large pharmaceutical organization managing multiple oncology products, it is essential to segment the supply chain and design tailored operating models through the road map. Additionally, the supply chain operating model strategy and design should be regularly evaluated and refined as the product portfolio and business evolve over time.
By designing a future-ready capability road map, life sciences organizations can fulfill the common and unique requirements of various therapies, from traditional chemotherapy to advanced immunotherapies. This proactive approach will help organizations adapt to the evolving landscape of cancer treatment, preparing them to manage future challenges.
2. Establish Strategic Partnerships
Building strong relationships with suppliers and service providers is crucial to enhancing supply chain resilience. In the rapidly evolving landscape of oncology, pharmaceutical organizations must prioritize the establishment of strong strategic partnerships with critical raw material suppliers, contract manufacturers, and logistics providers.
By fostering collaborative relationships, organizations can enable the timely availability of essential materials and maintain high quality standards throughout production. Strategic partnerships enable better communication, innovation, and agility in responding to market demands, ultimately leading to improved patient outcomes and a competitive edge in the oncology sector. Emphasizing transparency and shared goals can further strengthen these alliances, paving the way for sustainable growth and success in delivering lifesaving therapies.
Additionally, organizations should consider forming alliances with academic institutions and biotech firms to accelerate the discovery and delivery of cutting-edge cancer treatments. Collaborations can lead to shared resources, knowledge exchange, and innovative solutions that enhance supply chain capabilities.
3. Invest in Advanced Manufacturing Technologies
Organizations should invest in flexible manufacturing facilities equipped with digital and automation capabilities to accommodate the production of various oncology therapies. The manufacturing process for cancer therapies is complex, involving the synthesis of active pharmaceutical ingredients, formulation, and packaging, each requiring stringent quality control.
Given the wide-ranging nature of cancer treatments, each therapy and modality presents unique supply chain challenges that must be addressed to facilitate consistent availability and quality. For instance, chemotherapy drugs can be administered orally or intravenously, but their manufacturing process is complex and requires stringent quality control to handle hazardous materials and maintain proper storage conditions.
To meet these challenges, life sciences organizations must transition from pilot projects and proofs of concept to full-scale implementation of Industry 4.0 and digital manufacturing solutions. By leveraging advanced data acquisition, analytics, and decision intelligence technologies — such as control towers and digital twins — organizations can achieve higher quality, improved yield, and reduced production times. Real-time process data verification and in-line analytics focused on critical quality parameters exemplify how advanced digital technologies can enhance manufacturing.
In addition, organizations must transition from manual processes to automated systems that promote operational visibility and interoperability. This integration can enhance product quality and reduce lead times, ultimately improving the overall efficiency of the supply chain.
4. Develop Robust Cold Chain Logistics
With many oncology therapies requiring temperature-sensitive handling, organizations must invest in specialized cold chain logistics. This includes developing robust capabilities, both internally and with distribution and logistics service providers, to handle biologics, cell therapies, and cancer vaccines.
Cold chain logistics are critical for preserving the stability and efficacy of biologic products. For example, immunotherapy harnesses the body’s immune system to recognize and destroy cancer cells, requiring advanced biotechnological processes and a highly coordinated supply chain for the timely collection, modification, and delivery of cells. Employing strong cold chain logistics is essential for maintaining biologic products at the appropriate temperatures throughout the supply chain.
Organizations should invest in specialized storage and transportation solutions to maintain product integrity. Additionally, mid- to long-range planning processes should assess and define cold chain capacity requirements. Training staff and logistics partners in the specialized handling requirements for different oncology modalities, including radioactive materials and personalized cell therapies, is essential to compliance and safety.
Furthermore, organizations must develop contingency plans to address equipment failures, transportation delays, and other potential disruptions in the cold chain. By proactively managing these risks, organizations can enable the uninterrupted delivery of essential oncology therapies.
5. Leverage Data Analytics and Other Technologies
Life sciences organizations should harness data analytics to gain insights into supply chain performance, demand patterns, and inventory levels. Investing in blockchain, the Internet of Things, artificial intelligence (AI), and other digital technologies can drive supply chain visibility, traceability, and efficiency. These technologies empower organizations to monitor real-time conditions, track shipments, and predict potential disruptions. For instance, data analytics can provide insights into patient demand, allowing organizations to optimize inventory levels and reduce the risk of stockouts. By leveraging data analytics, organizations can optimize their supply chains, drive regulatory compliance, and integrate advanced manufacturing technologies, ultimately improving the availability and quality of cancer therapies worldwide.
Additionally, organizations should implement advanced traceability systems to track products throughout the supply chain, facilitating transparency and accountability. This is especially vital for personalized therapies, such as CAR T cells, where maintaining the chain of identity and custody is imperative to ensure patient safety and regulatory adherence.
6. Proactively Manage Supply Chain Risks
To build resilience, companies must conduct regular risk assessments to identify potential vulnerabilities within their supply chains and develop effective mitigation strategies. This proactive approach should encompass contingency planning for supply disruptions caused by critical suppliers or service providers, natural disasters, geopolitical events, and other unforeseen circumstances.
Organizations should diversify their supplier base, establish strategic partnerships, and maintain safety stocks of critical components and raw materials. With these measures, they can enhance their ability to respond swiftly to challenges, enabling the uninterrupted delivery of essential oncology therapies and safeguarding patient care.
7. Monitor Regulatory Compliance Continuously
Oncology companies must develop robust capabilities to continuously monitor the ever-changing global regulatory compliance requirements and effectively track and trace their complex products. Maintaining a comprehensive understanding of the regulatory landscape for each oncology modality is essential for compliance with local and international regulations.
This involves meticulous documentation, traceability, and reporting. Implementing advanced traceability systems is crucial to tracking products throughout the supply chain, thereby ensuring transparency and accountability. This is especially vital for CAR T cells and other personalized therapies that require maintaining the chain of identity and custody to drive patient safety and regulatory adherence.
8. Adopt a Patient-Centric Approach
Finally, organizations’ supply chain strategies must prioritize patient access to therapies. This includes focusing on timely delivery, adherence support services, patient assistance programs, and education. Establishing feedback mechanisms to gather insights from patients, healthcare providers, and other stakeholders can be invaluable. The resulting information can be used to continuously improve supply chain processes and address any complications promptly.
Conclusion
A well-coordinated and resilient supply chain is imperative to meeting the increasing demand for innovative cancer treatments and enabling the best possible patient care. As cancer rates continue to rise, it becomes even more vital for life sciences organizations to implement the abovementioned eight strategies. By doing so, they can enhance their operational efficiency, reduce costs, and improve patient outcomes, significantly contributing to the fight against cancer. And by addressing the complexities and unique requirements associated with various cancer therapies, organizations can establish highly reliable and resilient supply chains along with their ecosystem partners. Such a proactive approach will not only enhance the availability of lifesaving therapies but also transform cancer treatment and improve the lives of millions.
The views reflected in this article are the views of the author and do not necessarily reflect the views of Ernst & Young LLP or other members of the global EY organization.
References
- “Cancer Tomorrow,” International Agency for Research on Cancer, https://gco.iarc.who.int/tomorrow/en/dataviz/trends?multiple_populations=1.
- “Global Oncology Trends 2024: Outlook to 2028,” May 28, 2024, IQVIA Institute for Human Data Science, https://www.iqvia.com/insights/the-iqvia-institute/reports-and-publications/reports/global-oncology-trends-2024.
About The Author:
 Kerim Ozbilge is a principal in the Supply Chain & Operations Consulting practice at Ernst & Young LLP (EY U.S.) with more than 25 years’ experience managing full life cycle supply chain transformation programs. He’s passionate about helping his clients solve their toughest business challenges through supply chain operating model design and implementation including digital technology enablement.
Kerim Ozbilge is a principal in the Supply Chain & Operations Consulting practice at Ernst & Young LLP (EY U.S.) with more than 25 years’ experience managing full life cycle supply chain transformation programs. He’s passionate about helping his clients solve their toughest business challenges through supply chain operating model design and implementation including digital technology enablement.
