Wearable Injectors: An Alternative To Traditional Intravenous Administration
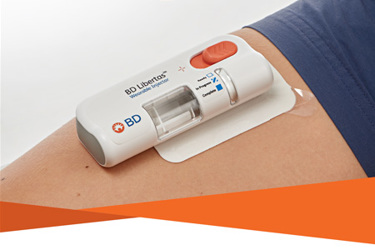
- The BD Libertas™ Wearable Injector is designed to deliver subcutaneous injections of large volume (2-5 mL or 5-10 mL) and/or high viscosity (up to 50 cP) fixed-dose complex biologics in home or clinical settings.1
- The 2-5 mL device was evaluated in an early feasibility clinical study with a viscous placebo for its functional performance and tissue effects, pain tolerability and overall acceptability among 52 healthy participants.*2
This successful early feasibility clinical study of the BD Libertas™ Wearable Injector demonstrated consistent functional performance as designed with broad subject acceptability across all demographic categories (age, gender, BMI) and injection sites tested with or without ambulatory movement during injection.
Results hold great promise for the future of subcutaneous chronic disease therapies, positioning the BD Libertas™ Wearable Injector as a viable alternative to traditional intravenous administration.*2
access the Infographic!
Log In
Get unlimited access to:
Trend and Thought Leadership Articles

Case Studies & White Papers

Extensive Product Database

Members-Only Premium Content

Welcome Back! Please Log In to Continue.
X
Enter your credentials below to log in. Not yet a member of Pharmaceutical Online? Subscribe today.
Subscribe to Pharmaceutical Online
X
Subscribe to Pharmaceutical Online
