What waits in the wings as therapeutic proteins face manufacturing limitations?

The market for therapeutic proteins has experienced higher growth rates than most other pharmaceutical segments. This growth is expected to continue and revenues from the therapeutic proteins are forecast to reach $32.5bn in 2005.
As the range of therapeutic proteins on the market continues to grow, the ability to manufacture sufficient protein to match demand will become a significant factor limiting sales. This is expected to encourage the adoption of new technologies to manufacture proteins and, alternatively, novel therapies that could replace the therapeutic proteins.
Datamonitor's report, Therapeutic Proteins: Key Markets and Future Strategies, reveals:
- Manufacturing sufficient therapeutic protein to meet patient demand, even without growth from further indications, is proving difficult
- Increasing demand for therapeutic proteins will drive greater penetration of transgenic production
- Longer term, gene therapy is expected to reduce the market demand for therapeutic proteins significantly
Manufacturing sufficient therapeutic protein to meet patient demand, even without growth from further indications, is proving difficult
At present, manufacturers are finding that demand exceeds supply for therapeutic proteins. For example, from January 1 2001, Immunex's Enbrel (etanercept) was in limited supply to patients: Immunex was forced to begin the "Enbrel Enrollment Program" to control supply to current users, and limit uptake by new patients. Only in April 2001 were new patients being accepted for Enbrel therapy.
This problem will get worse as development of further indications fuels expansion in the patient numbers requesting therapeutic protein based therapies. Immunex is unable to meet demand under its indication for moderate to severe arthritis, yet it is in clinical trials for the further indication of psoriatic arthritis. Therefore, manufacturing capacity may be harming sales in the present, and reducing the future potential of a drug through limiting research into new indications.
Datamonitor's primary research found that restricted manufacturing capacity is not limited to Enbrel, but is affecting proteins manufactured using yeast/bacterial fermentors and those produced with recombinant mammalian cell cultures. Central to this is the lack of investment in plant capacity, leaving companies that suffered from overcapacity in the first stages of the therapeutic protein growth with the opposite problem today. A typical 10,000 liter fermentor can produce only 200kg of protein annually, at a capital construction cost approaching $100m, yet 200kg is often insufficient to meet patient demand.
Increasing demand for therapeutic proteins will drive greater penetration of transgenic production
It is biotechnology that offers the solution to insufficient manufacturing capacity. Using transgenic animals and plants has the potential to increase production quantities relatively cheaply. Goats, such as those by Nexia and Genzyme Transgenics, can produce up to 1kg of protein per year in their milk. Therefore, a medium sized dairy herd has the potential to match the protein production of a typical fermentor facility. This technology is already available and could soon be producing therapeutic proteins approved for medical use.
In May 2000, the first product manufactured using a transgenic animal, Genzyme Transgenics' recombinant human antithrombin III (rhATIII), received orphan drug status for restoring heparin sensitivity. Although development was halted due to the cost of further clinical trials compared to the market size, Genzyme Transgenics' is continuing with pre-clinical studies for transgenic production of Centocor's monoclonal antibody Remicade (infliximab).
Production capacity may be limiting therapeutic protein potential at present. However, if companies such as Genzyme Transgenics can succeed in meeting FDA safety requirements for products manufactured using transgenic animals, this may not prove to be resistor of growth in the long term.
The figure shows the most significant drivers and resistors impacting the development of transgenic animal or plant production of therapeutic proteins.
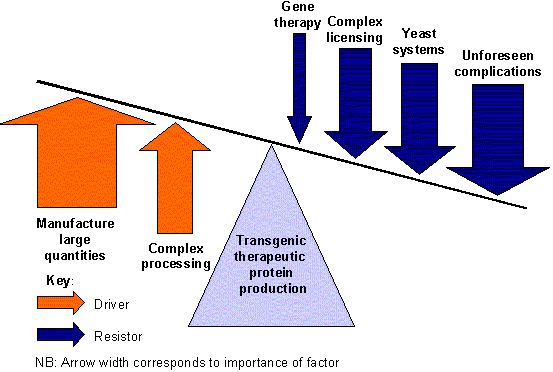
Datamonitor Analyst Oliver Sexton comments: "The increased use of transgenic production systems offers the potential to overcome the limitations of under-capacity for protein production and may finally drive the realization of biotechnologies full potential."
Longer term, gene therapy is expected to reduce the market demand for therapeutic proteins significantly
Although demand for therapeutic proteins is presently increasing and is forecast to break $30bn within five years, it is likely that, within the next 10 to 15 years, the market for proteins that replace or stimulate naturally circulating molecules will have significant competition. Two new types of treatment that are currently hitting the headlines, gene therapy and stem cell therapy, will have begun to impact the pharmaceutical market.
Gene therapy is able to modify and repair the genetic sequence responsible for a flawed protein and, by acting one stage earlier than a therapeutic protein, replace the need for substitution therapy. Stem cells can be used to grow replacements for damaged cells. Stem cell therapy could potentially treat degenerative diseases, such as arthritis, thus going one step beyond the reduction in symptoms that therapeutic proteins offer.
A market in which gene therapies are generating strong interest is cancer. For some types of cancer, defective genes are a major risk factor. For example, in breast cancer, approximately 10% of patients have been found to carry a mutation that is likely to lead to cancer. Replacing the defective gene can, in theory, help to cure the disease. Alternatively, gene therapies can be used in the treatment of cancer by introducing apoptosis genes that cause the death of targeted cells, or by introducing genes which, when expressed, cause the production of a cytotoxic protein. However, when gene therapy is used as a cure rather than a preventative therapy, as when treating cancer through cell apoptosis, it competes directly against monoclonal antibodies such as Rituxan and Herceptin. Therefore, when used purely as a cure, the impact of gene therapy may be more limited.
Gene and stem cell therapy may reduce the market for therapeutic proteins and prevent sales reaching their full potential, due to the extensive research that government and private organizations are devoting to these alternative therapies. Many executives involved with therapeutic proteins believe gene therapy will become a reality within the next 10 years and will significantly reduce the requirement for therapeutic proteins. Moreover, the successful launch of a gene therapy could spark a medical revolution similar to that began by the launch of recombinant insulin by Lilly in 1982.
Datamonitor Analyst Oliver Sexton comments: "Although the increased use of transgenic production systems would appear inevitable given the problems meeting existing demand, it may prove to be the Betamax of medicine and be overtaken by gene therapy or stem cell therapy."
For further information, contact Elisabeth Overend-Freeman at efreeman@datamonitor.com . Therapeutic Proteins is available from Datamonitor, priced at $5,800.
Datamonitor is an independent market analysis firm that publishes a wide portfolio of strategic business information. Datamonitor has expertise in the following industry sectors: Automotive & Transport; Consumer Goods; Financial Services; Healthcare; Industrial; Medical Devices; Technology. Datamonitor can be contacted at 212-686-7400 or visit www.datamonitor.com.
Source: Datamonitor
Subscribe to our free e-mail newsletter.
Click for a free Buyer's Guide listing.
