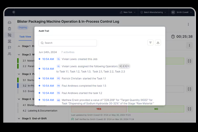
ABOUT ECOLAB LIFE SCIENCES
Ecolab Life Sciences provides advanced contamination control solutions and expertise in cleaning, disinfection and bio-decontamination to pharmaceutical production environments, globally. Supported by global technical and regulatory expertise, alongside dedicated account managers, Ecolab Life Sciences provides a world class service to help keep pharmaceutical operations GMP compliant and their products meeting the highest quality standards. Ecolab delivers enhanced value to customers, helping optimize production performance and mitigate risk.
With the addition of digital solutions and collaborative tools that proactively support our customers to continuously improve, manage change and achieve operational excellence, we help pharmaceutical manufacturers achieve consistent productivity, security and sustainability throughout their value chain.
Enhanced value: Global expertise, available regionally, applied to Ecolab’s cleaning and advanced contamination control solutions means customers have access to a comprehensive portfolio of high-quality cleaning and disinfection chemistry, equipment, and Bioquell vaporized hydrogen peroxide bio-decontamination.
Optimized production performance: Industry expertise across a wide range of critical manufacturing applications helps customers move towards maximum production uptime and productivity.
Mitigated risk: Expertise and global scale applied across our customers’ contamination-control operations help assist with business continuity through the worldwide availability of our solutions and knowledge of region-specific quality and regulatory requirements.
PRODUCTS AND SERVICES
EQUIPMENT
-
Create an aseptic workspace with Ecolab’s Bioquell Qube, a configurable isolator integrated with Bioquell Hydrogen Peroxide Sterilant*. From its unique design to rapid turnaround time, the Bioquell Qube provides the aseptic area you need to keep your research or production on track.
-
Reduce microbiological organisms on equipment, in transfer chambers, in filling line isolators and more with Ecolab’s Bioquell IG-2, a robust, fixed bio-decontamination system.
-
Do you need a reliable contamination control strategy in your lab or manufacturing facility, but lack the staff and equipment to carry it out? Bioquell Rapid Contamination Control Service from Ecolab is the answer.
-
Bio-decontaminate equipment, small areas, and large rooms too with Ecolab’s Bioquell L-4. This versatile, multi-purpose bio-decontamination system is easy to set up and operate.
-
Treat nearly any room or area in your facility with this mobile, scalable system. Ecolab’s Bioquell ProteQ features wireless communication technology, built-in aeration, and the option to add additional aeration capability.
-
Wall-mounted and compact, Ecolab’s Bioquell SeQure system helps protect your research and production facility from contamination risks.
DIGITAL
-
There's a need for continuous innovation to develop life-saving drugs and therapies. Explore the key outcomes of embracing Pharma 4.0 — and how they ladder up to drive critical business strategies.
-
Sterility testing is susceptible to contamination, resulting in costly false positives, batch failures, and production delays. Isolator technology offers a robust solution to mitigate these challenges.
-
Optimize pharmaceutical manufacturing with real-time cleaning data, audit readiness, and automated compliance. This end-to-end compliance platform empowers companies to meet stringent regulatory standards.
-
As the time, costs and complexity of cleaning validation challenges grow, savvy life sciences companies are building a competitive advantage around smarter approaches to cleaning validation.
SERVICES
-
In such a highly regulated environment, change is not undertaken lightly. As a result, a lack of internal resources can often obstruct the implementation of change.
-
An optimized and effective Contamination Control Strategy (CCS) helps you organization spend less time cleaning and more time manufacturing. Ecolab Life Sciences experts are dedicated to helping you drive the best possible strategy to meet and exceed the GMO Annex 1 CCS requirements.
-
From risk assessment to bio-decontamination solutions, drive continuous improvement in contamination control with tailored expert support.
CHEMISTRY
-
Ensure compliance, operational efficiency, and contamination control through tailored cleaning and disinfection solutions like validated programs, technical support, and sterile products.
-
Prepare for Annex 1 compliance with expert-led support in contamination control, featuring solutions that cover risk assessment, validation, and training to ensure cleanroom safety and compliance.
CONTACT INFORMATION
Ecolab Life Sciences
1 Ecolab Place
St. Paul, MN 55102
UNITED STATES
FEATURED INSIGHTS
CLEANING VALIDATION
-
Is your cleaning validation process truly aligned with current regulatory expectations? This checklist helps you implement a risk-based approach, focusing resources to effectively mitigate your highest-risk areas.
-
Effective small surface disinfection in cleanrooms requires adherence to detailed procedures and best practices. Learn about the critical steps, techniques, and validation needed to ensure product safety.
-
Implement a robust cleaning and sanitization program to ensure the safety and quality of personal care and cosmetics products while complying with global regulations.
-
Explore how to enhance product quality and safety in cosmetics manufacturing by standardizing cleaning procedures and emphasizing strict adherence to protocols.
-
Learn how implementing a systematic approach to cleaning validation can significantly enhance compliance and improve your audit readiness.
-
Unearth how adopting a risk-based approach to cleaning validation can enhance product quality, safety, and regulatory compliance in pharmaceutical manufacturing.
-
Learn about the technical nuances of Visual Residue Limits (VRLs) determination and explore strategies to implement robust VRL programs that meet regulatory standards and enhance manufacturing efficiency.
-
Discover how Ongoing Process Verification, guided by PAT and digital integration, ensures consistent cleaning efficacy, reduces risks, and optimizes production efficiency in the pharmaceutical industry.
INDUSTRY REGULATIONS
-
FDA 483 findings frequently cite a lack of data integrity, process adherence, and quality control. Find out why these issues persist and the significant risks they pose to pharmaceutical manufacturers today.
-
New EU rules tighten grip on contamination control for sterile drugs. Learn how to ensure your cleaning and disinfection meet the stricter standards.
-
The new Annex 1 regulations will significantly impact facilities and companies globally, requiring a comprehensive Contamination Control Strategy (CCS). Learn how to navigate these changes and ensure compliance.
DIGITALIZATION
-
Digital technologies are revolutionizing cleaning validation in decades-old pharmaceutical facilities. Learn about innovative non-intrusive methods to overcome the digital divide in legacy equipment.
PATHOGENS
-
Learn how effective disinfection solutions can ensure safety in pharmaceutical manufacturing by managing viral contaminants and enhancing patient protection.
STERILITY TESTING
-
Is your sterility testing process hindering your product release? Discover how the right approach can streamline your process, reduce false positives, and ultimately save you time and money.
VIDEOS AND WEBINARS

Choosing The Right Sporicide: Critical Factors For Robust Cleanroom Contamination Control
Choosing the best sporicide for your cleanroom involves evaluating factors like surface format, contact time, and safety. Learn the criteria to select an effective and robust agent for contamination control.

How Digital Cleaning Validation Can Simplify Audit Readiness And Improve Compliance
Discover how to enhance audit readiness and improve compliance by shifting to a digital cleaning validation program. Learn to determine worst-case products and maximum safe carryover limits.