
LIQUID DOSE FILLING

A Data-Driven Approach To Container Closure Integrity
Gain confidence in your container closure system selection by utilizing a stepwise and data-driven approach to predicting and verifying container closure integrity.

Early-Stage Considerations For The Selection Of Pre-Fillable Syringes When Developing A Vaccine
Get an overview of the strategy to speed up the migration from a vial to a pre-fillable syringe. Explore the main considerations in meeting and overcoming PFS-drug challenges when developing a vaccine.

Mycap® Bottle Closures – Aseptic Fluid Transfer Systems For Bottles
The innovative MYCAP bottle closure system provides more options for aseptic fluid transfer in bottles and rigid containers. The elastomeric closure makes it a truly universal closure system.

The Successful Implementation Of A New Aseptic Filling Line
Discover how a Quality by Design approach can streamline the development of new sterile product filling lines, featuring a real-life example illustrating its effectiveness.
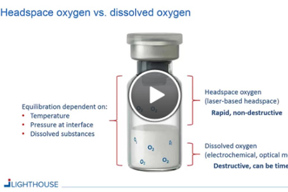
Determining And Controlling Oxygen Levels In Sensitive Formulations
This webinar will review how oxygen levels in finished parenteral drug containers can be determined and controlled throughout the product life cycle by using laser-based headspace analysis.

Alba® Syringe Platform: Ensuring Stability For Sensitive Drugs
Check out this exciting video showcasing the key advantages of Alba®: innovative coating technology that reduces potential interaction between the drug and container surface.
Mitigating Ultracold Storage And Transport Risk With CCIT
Dr. Derek Duncan, Director of Product Lines, Lighthouse Instruments, and Brandon Zurawlow, Chief Scientific Officer, CS Analytical, discuss a program for generating packaging data for deep cold storage products.
Consistent, Reliable, High-Quality Components
Discover a premium line of components for vials, prefilled syringes, and cartridge systems developed and refined to provide best-in-class levels of quality, performance, and risk mitigation.

Driving Excellence In Aseptic Fill-Finish: A Strategic Partnership With Stäubli Robotics
See how intelligent automation and purpose-built robotics deliver contamination control and operational excellence in demanding sterile manufacturing environments.

EZ-fill Smart™ Enhancing Aseptic Fill-Finish Efficiency In Pharmaceutical Manufacturing
Discover how the EZ-fill Smart system, integrated with Groninger technology, enhances aseptic fill-finish processes, improving efficiency, sterility, and scalability in pharmaceutical manufacturing.

Selecting A Prefillable Syringe System With Confidence
Discover an advancement in the prefillable syringe market, uniquely integrating the syringe barrel, plunger, and needle shield/tip cap into a fully harmonized, verified system from a single supplier.

Selecting Container Closure Components: Extractables And Leachables
Primary packaging components require a thorough assessment to ensure safety and efficacy. Plan ahead and start your assessment early to mitigate your risks, delays, and out-of-pocket costs.

Using Positive Controls In Container Closure Integrity Studies
This webinar describes the use of positive controls as an important element of CCI studies designed to validate packaging components for CCI or to qualify processes for producing good CCI.

Ensure Speed And Success With Your Drug Delivery Project
Hear from experienced professionals in the drug delivery space on how to ensure success and speed-to-market by establishing a firm foundation when working with a CMO.

EZ-fill® Kit: RTU Packaging Solutions For Fast Access To Small Volumes
Discover the EZfill Kit in action—an innovative solution designed to streamline and enhance precision in liquid handling, optimizing efficiency for pharmaceutical and bioprocess applications.
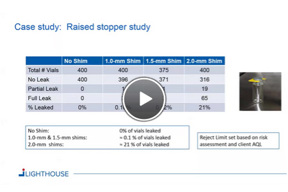
CCI Testing And Revisions To EU Annex 1
The new language in EU Annex 1 will likely have a significant impact on your CCI testing practices. In this webinar CCI testing strategies and the proposed revisions to EU Annex 1 are discussed.
EU Med Device Regulations: Ensuring Compliance For Integral Combination Products
Review the EU Medical Device Regulation (MDR) changes, specifically with respect to integral combination products, and examine the technical documentation packages supporting functionality and content.
