
LOGISTICS

Thermo Fisher Scientific Single-Use Global Manufacturing Network
We have implemented a multi-tier strategy to improve the resiliency of our supply chain and created the largest networked single-use manufacturing organization in the world.

Supporting The Global Biopharma Industry With Single-Use Capacity Expansion
Explore Cytiva’s brand new single-use production facility in Wales, UK. This facility forms part of Cytiva’s >$1.5 billion investment in increased capacity to support the growing needs of the biopharmaceutical industry and to increase regional supply.

Early-Stage Considerations For The Selection Of Pre-Fillable Syringes When Developing A Vaccine
Get an overview of the strategy to speed up the migration from a vial to a pre-fillable syringe. Explore the main considerations in meeting and overcoming PFS-drug challenges when developing a vaccine.

Robust And Consistent Frozen Storage And Shipment
Learn how a reliable cold chain operation requires the right commercial scale solution, from controlled bag film production to a unified process including reliable control mechanisms.
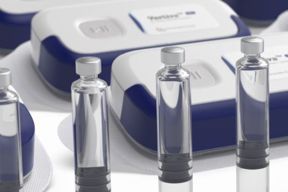
Highly Flexible And Customizable On-Body Delivery System Platform
Learn how Vertiva® can enable delivery of micro-precision basal doses and full-content bolus injections, resulting in a highly flexible, customizable platform suitable for a wide range of therapies.

Keep It Cool: Cellular Cryopreservation For Clinical Applications
Cryopreservation is essential for storing stem and T cells for therapy but poses challenges like low survival and function loss. Watch to explore optimization strategies and solutions.

The Way Forward: Supply Chain Solutions
Learn how to build a resilient supply chain to navigate today's complex environment. Expert speakers share insights on optimization, innovation, and strategic planning to meet evolving market conditions.

Singapore Single-Use Site Capacity Expansion
Thermo Fisher Scientific is expanding our single-use manufacturing network globally, an expansion site closer to Asia Pacific region
