
PRODUCTION
How To Select Your DSP Chromatography Technology
Watch to explore the differences between the conventional single-column batch method and the multi-column approach for mAb capture.
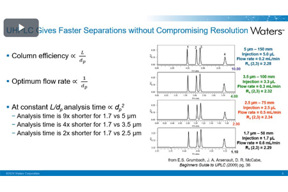
UPLC Columns: Fundamentals, Chemistries, And Selected Small-Molecule Applications
Discover the fundamentals of UPLC columns and their diverse chemistries. Learn more about versatility in separating small-molecule analytes using reversed-phase and hydrophilic interaction techniques.

The Route To Faster Microbial Quality Control
Join product and application experts Anne-Grit Klees, Carine Krebs, and Esther Welterlin to discover the benefits of using a new rapid microbial detection platform.
Strategies For Scaling Up mRNA Manufacturing
The clinical promise of mRNA therapeutics and vaccines is clear, but compared to monoclonal antibodies or viral vectors, their novelty also brings new challenges to the manufacturing process.

Solving The Analytics Bottleneck In Cell Line Development
Discover a fully automated, benchtop, walk-away system for accelerated and efficient cell culture sample preparation. Learn how this system clarifies and purifies mAbs in one step without sacrificing quality.

How To Select Your DSP Chromatography Technology?
Making the switch from single batch to multi-column chromatography can dramatically reduce resin utilization, costs and eliminate bottlenecks without sacrificing quality. This animated feature compares the traditional, single batch approach to a multi-column alternative f...

Developing Modular Facility Proof-Of-Concept For Multi-Modal Bioprocessing
Learn about the innovative solution of designing and constructing manufacturing facilities using predesigned and prefabricated elements.

Faciliflex Cleanroom Module For Therapeutic Drug Manufacturing
The Faciliflex Module is a transformational new concept in designing cleanrooms for the production of lifesaving therapeutics.

Accelerating Progress: Key Considerations For CDMOs To Maximize Productivity And Scalability
Due to the urgent demand for vaccines and treatments, the biopharmaceutical industry is experiencing disruptive changes and increased demand on resources that are requiring a significant operational transformation. To accelerate the delivery of life-saving therapeutics, i...

Improve The Bioavailability Of Poorly Soluble Drugs
KinetiSol is a fusion-based, solvent-free process that utilizes frictional and shear energies — in a fraction of the time of other amorphous solid dispersion technologies.

Rapid Process Development And Technical Support For AAV Scaleup
Accelerate your AAV production journey. Learn how rapid process development and expert support can streamline your path from vial to purified bulk, ensuring scalable and efficient manufacturing.

Meet The Experts: Part 1 - Process Analytical Technology (PAT)
Watch part 1 of this 4-part interview series to learn about process analytical technology (PAT) from expert Stacy Shollenberger.

Strategies And Emerging Technologies For mAb Capture
Explore opportunities with the introduction of high-capacity protein A resins, how and when multicolumn chromatography is beneficial, and how emerging technologies can address bottlenecks.

Transform Your Chromatography Processes With An Innovative Approach
Learn how to achieve better resin utilization, minimize disruption to existing methods, and align your strategy with unique process goals.

Redefine Your Oligonucleotide Manufacturing
Streamline oligo production and scale confidently. Explore high-performing, purpose-built automated equipment designed to ensure safety, efficiency, and purity, from synthesis through concentration.

The Fusion-Based, Solvent-Free Process Disrupting Spray Drying
Review a commercially-ready process capable of reproducing spray-dried dispersions with less manufacturing complexity and environmental impact, and out-designing and outperforming spray drying.

Single-Use Technology For Quality mRNA Manufacturing
Learn how single-use technologies deliver important benefits for mRNA manufacturing, including increased speed, quality, flexibility, and adaptability to different dosages and scale-up strategies.
Using An Adherent Platform To Scale-Up An Upstream Viral Vector Process
Gain insight into a helper-dependent adenovirus (HDAd) process that covers everything from bench-scale and optimization to tech transfer.

Thermo Fisher Scientific North America Single-Use Technologies Capacity Expansion
Thermo Fisher Scientific is expanding our single-use manufacturing network globally. Some of the largest investments are taking place at our existing Logan, Utah, and Millersburg, Pennsylvania, sites. We are adding space for clean rooms for the manufacturing and assembly ...

Analytical Solutions To Help Accelerate Bioprocessing Success
Unlock the power of data-driven bioprocessing. Discover how advanced analytics can optimize your cell culture process, improve performance, and accelerate success.

Break Free From The Protein A Low-Productivity Paradigm
Protein A resins, with low flow rates and mass transport, lead to low productivity. Discover a solution that removes the bottleneck, offering a disposable alternative for mAb capture.

Scaling Up: Increasing Production Capacity While Reducing Costs
Medical device companies must meet the demand plan for their devices while managing production costs. Gain valuable insights for scaling up production capabilities efficiently and cost-effectively.

Bioburden And EU GMP Annex 1: Risk Assessment, Reduction, And Testing
Experts on regulation, filtration, sampling, and quality control discuss risk assessment and filtration-based strategies to reduce bioburden.

Small-Scale Oligonucleotide Synthesizer That Supports Scale Up
Learn about a small oligonucleotide synthesizer with big impact that features a user-friendly interface, compact footprint, and more. This small scale system is user friendly and supports scale up.

Challenges And Solutions In Aseptic Connection And Disconnection
Progressive biomanufacturing processes require advanced tools to reliably connect and disconnect components. Here, we show a wide range of connection and disconnection options.

Thermo Fisher Scientific China Single-Use Technologies Capacity Expansion
Thermo Fisher Scientific is expanding our single-use manufacturing network globally. With continuous growth in China’s population, we have seen an increase in demand for human therapeutics and vaccines and an even greater need for investment in public health and saf...

Plasmid DNA Downstream Purification Process
Large scale production of plasmid DNA requires an in-depth understanding and optimization of the entire downstream process. Explore a complete solution, from harvest to bulk filtration.

Technology To Overcome Absorption Hurdles And Solubility Limitations
For poorly soluble drugs, overcoming absorption hurdles can hinder development. Explore a solution that empowers researchers to overcome solubility limitations and accelerate drug development.

Intensification Strategies For Upstream, Downstream Processes
How do you know if an intensification approach will provide advantages? Three process intensification experts show how various intensification approaches affect outputs using different scenarios.

How mRNA Manufacturers Disrupt The Industry
Learn how biopharmaceutical companies can apply proven strategies to overcome hidden complexities in mRNA production, and discover solutions that can help you rapidly design and build a SU facility.
