
QUALITY ASSURANCE
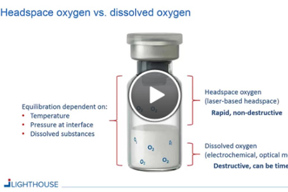
Determining And Controlling Oxygen Levels In Sensitive Formulations
This webinar will review how oxygen levels in finished parenteral drug containers can be determined and controlled throughout the product life cycle by using laser-based headspace analysis.

A Rapid Approach For Moisture Determination Of Lyophilized Product
Explore the limitations of traditional moisture determination techniques, an innovative approach using laser-based headspace analysis, and real-world case studies using this non-destructive method.

Airflow Visualization Studies: The Impact Of Annex 1 On Sterility Assurance
Airflow visualization techniques like Smoke Studies and CFD analyses are crucial for contamination control, aiding cleanroom qualification, environmental monitoring, and optimizing contamination control strategies under Annex 1.

Navigating GxP Compliance Challenges
Learn how to select and manage trusted software suppliers to streamline your digital transformation, mitigate risks, and ensure regulatory adherence. Watch to discover best practices and real-world examples.

The Pathway To Operational Readiness
Ready to ensure your facilities, systems, and teams are prepared for success from Day One? Discover a proven framework to accelerate operational readiness, mitigate risks, and achieve sustainable results.
Meet The Experts: PAT, Raman Spectroscopy, And Chemometrics
Gain a better understanding of process analytical technology (PAT), Raman spectroscopy, chemometrics, and Raman applications in bioprocessing.

Meet The Experts: Part 1 - Process Analytical Technology (PAT)
Watch part 1 of this 4-part interview series to learn about process analytical technology (PAT) from expert Stacy Shollenberger.

Using Positive Controls In Container Closure Integrity Studies
This webinar describes the use of positive controls as an important element of CCI studies designed to validate packaging components for CCI or to qualify processes for producing good CCI.

Revolutionizing Contract Manufacturing With A Unified QMS And MES Platform
Watch to gain expert insights on utilizing a closed-loop, single-platform approach and explore practical examples and data showcasing the advantages of integrating your QMS and MES.

Compressed Gas Risk Assessment: A Significant Step In Your CCS
Discover the importance of compressed gas monitoring in ensuring product quality and compliance with EU GMP standards.

Overcome The Hidden Complexities Of mRNA Process Development
Explore the challenges behind the perceived simplicity of mRNA process development, as well as characterization strategies and models for predicting process outcomes at early stages.
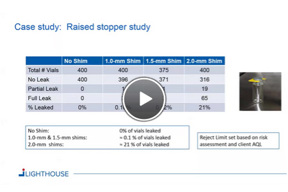
CCI Testing And Revisions To EU Annex 1
The new language in EU Annex 1 will likely have a significant impact on your CCI testing practices. In this webinar CCI testing strategies and the proposed revisions to EU Annex 1 are discussed.
