API Manufacturing And Material Handling Equipment
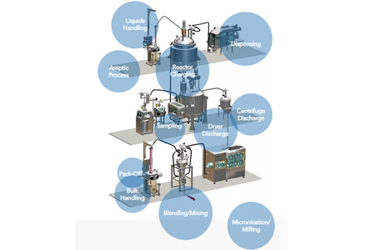
Active pharmaceutical ingredient (API) manufacturing requires powder handling in a GMP and contained manner. Similar to chemical manufacturing, operations like charging powders into a chemical reactor, emptying a centrifuge or charging and discharging a dryer are standard processes.
Dec offers innovative powder handling solutions from the raw material handling to the intermediate product reprocessing and to the final packing step under a safe and controlled atmosphere.
One of the typical steps in pharmaceutical manufacturing is to accurately dispense various components before blending them together.
The various powders may come in different packaging with various levels of toxicity and must be dispensed in a controlled environment excluding any exposure risks of the operator.
Such activities are still too often carried out in an open manner inside a Laminar Flow booth with issues for the operator due to strenuous manual handling, dust exposure and risks of cross contamination or inside an isolator when handling highly potent APIs with limitations as to the amount of powder which can be handled.
