Building Safety Into Isolator Controls Systems
Source: Dec Group
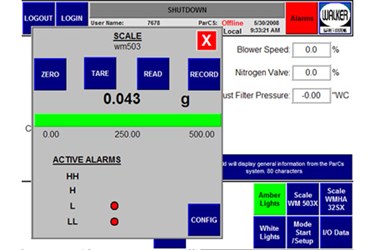
As isolators become more customized to meet the requirements of specific Pharmaceutical processes, the controls systems have been challenged to provide more safety and flexibility.
A process may require the isolator to have inflatable gaskets and provide oxygen monitoring. To meet the challenges, Walker Barrier Systems has designed a controls system that provides:
Automated start up sequence
- It is important to have an automated start up sequence to be certain that the isolator is sealed and in leak tight. This will assure the operators safety.
- Door seal test and pressure decay test is automatically performed before the isolator goes into “run” mode.
access the Case Study!
Log In
Get unlimited access to:
Trend and Thought Leadership Articles

Case Studies & White Papers

Extensive Product Database

Members-Only Premium Content

Welcome Back! Please Log In to Continue.
X
Enter your credentials below to log in. Not yet a member of Pharmaceutical Online? Subscribe today.
Subscribe to Pharmaceutical Online
X
Subscribe to Pharmaceutical Online
