Developing Mobile Continuous Process Technology: A Collaborative Innovation Case Study
By John Mulgrew, Centre for Continuous Manufacturing and Advanced Crystallization (CMAC), University of Strathclyde
In Part 1 of this two-part article, we explored the role of open collaboration in the future pharmaceutical manufacturing innovation. Part 2 provides a specific example of how such collaboration is already taking place, through a unique industry-academic partnership.
The REMEDIES (RE-configuring MEDIcines End-to-end Supply) project launched in 2014. The project is led by GlaxoSmithKline (GSK), with academic research partners University of Cambridge’s Institute for Manufacturing (IfM) and University of Strathclyde’s Centre for Continuous Manufacturing and Crystallisation (CMAC) leading the active pharmaceutical ingredient work stream, aka “App A.” It brings together key players in the end-to-end supply chain, including major contract manufacturing organizations, large pharmaceutical companies, and equipment manufacturers, along with regulators, academia, knowledge transfer networks, and healthcare providers.

Figure 1: REMEDIES project partners
The approximately £23 million ($29 million), U.K.-based project, due to be completed in March 2018, has several technology-based application projects underpinned by two platform projects (refer to Figure 2).
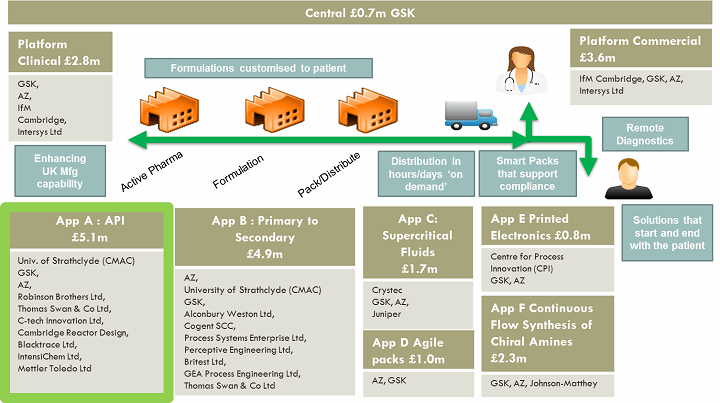
Figure 2: REMEDIES overview
The project involves several interrelated application work streams, including App A, which covers active pharmaceutical ingredients and registered starting materials portion of the supply chain. App A’s focus is developing mobile continuous API process equipment with open access to end-user partners. The result will be an asset network for use by CMOs and primes that otherwise would not be commercially available because the components are too costly or risky to develop by an individual company.
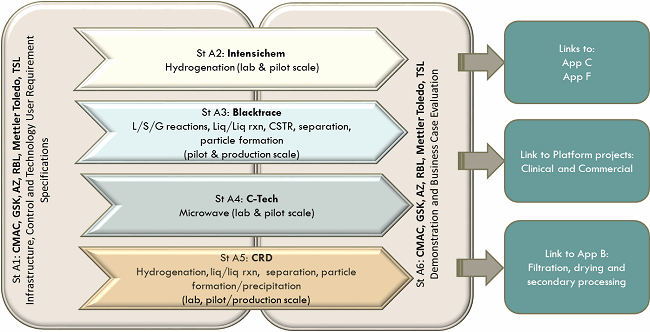
Figure 3: Overview of App A work stream
CMAC is leading App A with a vision of accelerating the adoption of continuous manufacturing processes, systems, and plants to produce high-value, high-quality pharmaceutical and fine chemical products more sustainably and at lower cost.
This collaboration consists of end users (GSK, AZ, Robinson Brothers Ltd., and Thomas Swan & Co. Ltd.), technology providers/manufacturers (Cambridge Reactor Design, Syrrius Blacktrace, C-Tech, and Intensichem), and oversight by CMAC. Funding is split between industry and government sources, roughly on a 50:50 basis, with industry paying for the work and subsequently being reimbursed based upon preapproved grant funds.
Here is what each partner is contributing to the collaboration:
CMAC is housed within the recently opened £90 million Technology and Innovation Centre at University of Strathclyde. It is making significant contributions to the pharmaceutical manufacturing industry through:
- Application of new technology
- New applications of existing technology
- Advances in manufacturing technology
- Advances in equipment design technology
- Understanding of critical process parameters and material characterisation
CMAC is providing technical guidance, knowledge exchange, and in some cases testing technologies being developed within App A.
GSK, AZ, Robinson Brothers Ltd., and Thomas Swan & Co. Ltd. are the end users and are providing high-level guidance and specifications for the new technology platforms being developed within App A. Technology platforms developed by the technology partners will be tested using end-user chemistries and in some cases tested at end-user manufacturing sites. The equipment being developed will help manufacturing companies to make the switch from batch to continuous manufacturing.
There are four technology partners, each developing different mobile continuous manufacturing equipment focused on active pharmaceutical reactions:
Cambridge Reactor Design (CRD) is developing three technology platforms – continuous crystalliser, continuous cascade mixed reactor, and tubular baffled reactor.
The continuous crystallisation reactor (reactions/processes, liquid/solid processes, particle formation & control) is large lab/manufacturing scale. CRD is designing and constructing a mobile continuous crystallization platform capable of being located within a GMP facility.
CRD is developing cascade mixed tank reactor geometry for continuous high-pressure catalytic hydrogenation, reactions, and processes (liquid/solid/gas) at large lab and pilot/manufacturing scales.
A tubular and baffled reactor for continuous chemical manufacturing, reactions, and processes (liquid/solid, separation, crystallisation, particle size control) at large lab and pilot/manufacturing scales is also being deployed.
Blacktrace is developing a modular system that can be easily reconfigured for multiple applications, including reactions and processes involving liquids, solids, and/or gases, separation, and particle formation at pilot/manufacturing scale. This new pilot/manufacturing scale kit is a larger version of the Blacktrace Asia Series of lab scale equipment but with the added benefit of equipment for potentially explosive atmospheres (ATEX), GMP, and good automated manufacturing practice (GAMP) compliance. This platform will improve upon the capabilities currently available to the larger-scale pharmaceutical production industry. The new platform is called the Titan Series and was launched at the P-MEC in Barcelona, Spain, in October of last year.
Intensichem is developing a transportable and scalable continuous flow, high performance, fixed-bed hydrogenation system. A fume cupboard-based system for early stage development trials has been completed. Design for a plant-hardened ATEX rated manufacturing system is underway that is based on a 10-foot transportable shipping container with reaction time in seconds for increased yields, reduced impurity formation, and significant potential for exploring new industrial chemistry. Equipment already developed includes Hydrogenator Lab v1, a 100bar and 1L/hour maximum flowrate, proof-of-concept tool, currently in use. Equipment under design and construction includes a hydrogenator at pilot/manufacturing scale capable of 12L/hour flowrate, 200bar maximum. Following on from this will be a Hydrogenator Lab v2 capable of 700bar and 0.3L/hour maximum flowrate, which will enable chemistries currently out of reach of pharmaceutical manufacturers.

Figure 4: Intensichem continuous hydrogenation platform — progressing from lab concept to industrial application
C-Tech provides expertise on microwave reactions and is producing a microwave reactor at pilot/manufacturing scale for chemical processes of reactions that are low yielding or energy demanding using conventional heating (liq/liq reactions and light slurries). This includes demonstrating scale-up of industrial processes using continuous flow microwave chemistry, providing an improvement in yield and/or energy efficiency to end-user processes via transfer to continuous flow microwave chemistry, and Integrating in-line analytical into the continuous flow microwave reactor to monitor reactions.
The technology partners are also working with Mettler-Toledo on integrating process analytical technology (PAT) to their platforms for better control and predictability.
Impact Of Collaboration
The impact of a collaboration like this is measured several ways, depending upon who is asking the questions.
For government funders, the impact is measured in GVA, jobs safeguarded, and jobs created, which can be seen through restoration of high-value manufacturing employment. Long-term benefits for government include the availability of higher-quality drugs at reduced cost and without shortages.
The impact for large pharmaceutical companies and contract manufacturing organizations is a broadening of their ability to manufacture in ways never seen before, resulting in lower up-front capital costs of processes, higher quality and higher yield than traditional manufacturing practices, and lower cost-to-benefit ratio, e.g., de-risking of investment. Another more difficult to measure benefit is the speed at which innovation can be achieved, as going it alone usually means much slower progress.
Ian McCubbin, GSK’s SVP of global manufacturing and supply, said: “The REMEDIES project is already playing a significant role in our work to transform the medicines supply chain,” and “GSK is particularly proud of the collaboration it has created, between leaders in the pharmaceutical industry, our partners and academia. We are confident that the project will ultimately result in real benefits to patients.”
 Chris Jones, vice president and global head of pharmaceutical development, AstraZeneca, said, “I am delighted that AstraZeneca is a key member of Project REMEDIES. The U.K.’s pharmaceutical industry is a key asset, and continued investment in the science and technology that underpins our industry is vital. Project REMEDIES brings together world leading scientists and engineers from major pharmaceutical companies such as AstraZeneca and GSK, from leading academic institutions, and from cutting-edge technology companies to work on a focused program of activities that will stimulate creative solutions and innovation.”
Chris Jones, vice president and global head of pharmaceutical development, AstraZeneca, said, “I am delighted that AstraZeneca is a key member of Project REMEDIES. The U.K.’s pharmaceutical industry is a key asset, and continued investment in the science and technology that underpins our industry is vital. Project REMEDIES brings together world leading scientists and engineers from major pharmaceutical companies such as AstraZeneca and GSK, from leading academic institutions, and from cutting-edge technology companies to work on a focused program of activities that will stimulate creative solutions and innovation.”
Technology providers will gain access to customers and invaluable input from end users into the design of new technology platforms that will be industrially relevant. For the technology companies, which are usually small to medium enterprises (SMEs), collaborations can provide the capital assistance necessary to innovate and develop new and novel technologies. “Through the scientific resources and experience offered by the REMEDIES consortium and the guidance of CMAC in App A, we have expanded our developed technology products portfolio and have a road map for further developments to meet the challenges of a rapidly expanding market,” reflected Dr. Benjamin Taylor, product manager at Blacktrace.
Lessons Learned
Public-private collaborations like REMEDIES are beginning to build trust between the various stakeholders. It takes time and effort -by organizations, especially as to understanding the tangible benefits for each partner, aligning objectives, and establishing how intellectual property will be handled. Other challenges to be mindful of are lack of standardization, communication channels, and logistics. Not having agreed-upon standards of data collection, data storage, and control system strategies can detract from the focus of the collaboration. Working out how collaborations will efficiently and effectively meet is greatly helped by today’s web conferencing, but standards of communication must be clearly defined.
An area that is rich in opportunity is at the interface between academic institutes, where much of the innovation is happening, and SMEs that are advancing technologies along the technology readiness continuum.
No matter what drives your company to innovate, collaborations are a cost-effective and impactful way to accelerate innovation. Start by evaluating the nature of your company, looking at how you want to innovate, and then reach out to people and organizations with the right skill sets to start the conversation.
The advantage of collaborative innovation is that, the more external parties involved in the innovation process, the better the overall quality of the resulting product. The positive outcomes of collaboration can be summarized as:
- Cost reduction: Fewer resources required for research and development
- Improvement in development & innovation productivity: Involvement with outside complementary knowledge providers will advance innovations.
- Accelerate time-to-market: New technology brought to bear sooner
- Increase differentiation: External and internal sources of knowledge and technical expertise can produce technically superior products that can help distinguish the organization from its rivals.
About The Author:
 John Mulgrew is industry collaboration project manager at CMAC – Strathclyde University’s Centre for Continuous Manufacturing and Advanced Crystallization. He has spent most of his career as a consulting engineer working with pharmaceutical and life sciences organizations to build large-scale manufacturing facilities. John’s studies to complete an MBA in corporate innovation led him to pursue his interest in innovation. He has brought his experience from the pharmaceutical industry together with his project management skills to guide multi-partner, pre-competitive collaborations. He is also working with the Centre for Process Innovation (CPI) to bring the Medicines Manufacturing Innovation Centre (MMIC), an open innovation center dedicated to small molecule pharma manufacturing, to Scotland.
John Mulgrew is industry collaboration project manager at CMAC – Strathclyde University’s Centre for Continuous Manufacturing and Advanced Crystallization. He has spent most of his career as a consulting engineer working with pharmaceutical and life sciences organizations to build large-scale manufacturing facilities. John’s studies to complete an MBA in corporate innovation led him to pursue his interest in innovation. He has brought his experience from the pharmaceutical industry together with his project management skills to guide multi-partner, pre-competitive collaborations. He is also working with the Centre for Process Innovation (CPI) to bring the Medicines Manufacturing Innovation Centre (MMIC), an open innovation center dedicated to small molecule pharma manufacturing, to Scotland.
In addition to his work at CMAC, John enjoys lecturing the project management module for the M.Sc. program in pharmaceutical manufacturing at University of Strathclyde, as well as guest lecturing on project management for various other universities courses.
You can connect with John on LinkedIn and find more information on REMEDIES here.
