Ensuring Safe Handling Of High Potency Products
By Gabriela Mikhaiel, Dec Group Marketing, business marketing manager for products and services, Dec Group
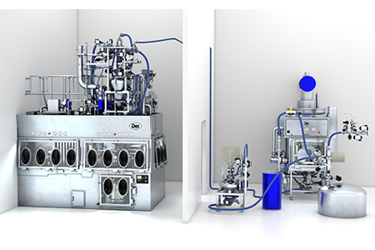
The pharmaceutical industry is developing at a faster pace than ever before. New drugs and forms of therapies, persistent market growth, Industry 4.0 and the current COVID-19 pandemic are synonym to enormous challenges for manufacturing companies.
Due to the high potency of the pharmaceutical active ingredients (APIs), machine operators have to be protected and while at the same time, humans are a high source for product contamination, the key to a successful operation lies in reliably engineered technologies to bring forth equipment capable to offer suitable containment solutions to protect people and products from each other.
In pharmaceutical manufacturing facilities, isolators are becoming increasingly important as they can achieve the desired level of protection and security.
This article presents a fully integrated highly complex isolator and process equipment solution offering high safety, efficient control and cleaning features as well as full compliance in terms of manufacturing regulations.
Get unlimited access to:
Enter your credentials below to log in. Not yet a member of Pharmaceutical Online? Subscribe today.
