Helium Leak Detection Vs. Dye Leak Testing
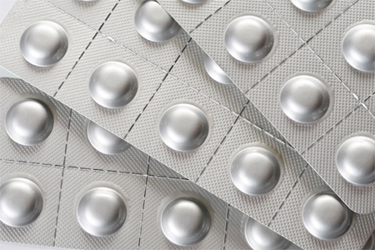
A global pharmaceutical entity was experiencing early package failures from stability studies for a new drug product. The package was a cold-formed foil blister card that contained a wafer type product. Samples were leak tested using dye ingress methods, and no leak failures were noted. However, packaged samples failed at an alarming rate after a brief period under accelerated storage conditions. Learn about the solution and the results obtained from this case.
access the Case Study!
Log In
Get unlimited access to:
Trend and Thought Leadership Articles

Case Studies & White Papers

Extensive Product Database

Members-Only Premium Content

Welcome Back! Please Log In to Continue.
X
Enter your credentials below to log in. Not yet a member of Pharmaceutical Online? Subscribe today.
Subscribe to Pharmaceutical Online
X
Subscribe to Pharmaceutical Online
