Is It Time To Stop Using Mean Kinetic Temperature (MKT) In Pharma Storage & Transport?
By Tobias Kuners of Koenders, managing consultant, Tob Management
 We have come a long way in condition monitoring and control in pharmaceutical storage and transport. Fifty years after John D. Hayes’ frequently cited article,1 I am proposing moving away from the concept of MKT as the tool to evaluate temperature excursions in transport and storage of drug products. The original purpose of Hayes’ article in 1971 was to address climate-based temperature variations in uncontrolled pharmaceutical storage to relate to a single temperature that could be substituted for product expiry testing. Explained differently, MKT can be determined by calculating a “virtual temperature” that would consider expected temperature variability in a given geography. The article clearly states that for those products whose loss rate constant is related to temperature by the Arrhenius relationship, a single virtual temperature can be determined at which the loss rate is equivalent to that of this changing pattern of temperature.
We have come a long way in condition monitoring and control in pharmaceutical storage and transport. Fifty years after John D. Hayes’ frequently cited article,1 I am proposing moving away from the concept of MKT as the tool to evaluate temperature excursions in transport and storage of drug products. The original purpose of Hayes’ article in 1971 was to address climate-based temperature variations in uncontrolled pharmaceutical storage to relate to a single temperature that could be substituted for product expiry testing. Explained differently, MKT can be determined by calculating a “virtual temperature” that would consider expected temperature variability in a given geography. The article clearly states that for those products whose loss rate constant is related to temperature by the Arrhenius relationship, a single virtual temperature can be determined at which the loss rate is equivalent to that of this changing pattern of temperature.
However, it was not until 2001 that J. Taylor of the Medicine Controls Agency2 presented a different application for MKT. This was quickly adopted by industry as a tool to evaluate the impact of temperature excursions on drug product quality and turned out to be a landmark change in the application of MKT, as it is still used to this date. Regulatory bodies propose and use it as their guidance for industry. Interestingly, in the original article by Taylor, it is stated: “Strict conditions should be applied to the use of MKT. It is applicable only to storage of products under controlled room temperature conditions (i.e., those labeled ‘do not store above 25C’).” Further in the same article, Taylor states, “MKT should not be used to compensate for poor temperature control of storage facilities.” To date, MKT continues to be proposed in USP chapters <659> and <1079> and is presented as an isothermal storage temperature that simulates the non-isothermal effects of storage temperature variation. However, there is too little or no emphasis on the physics or chemistry behind the impact of temperature excursions on biological drug products and other contemporary drugs.
In this article, I propose to take a modern approach to temperature excursions in storage and transport.
Storage and transport are both holding functions, where the first is stationary and the second is mobile. Regarding drug products’ temperature exposure, there is no rationale to treat storage any differently than transport. In exceptional cases, such as vaccines that need to be stored at very low temperatures (< -70°C), arguments are being made that transport at -20°C is acceptable, only because transport at temperature below -70°C is complex. However, even at such low temperatures, tests need to be done to prove the validity of the statement.
Limitations To Using MKT
The following equation was developed for a “virtual temperature,” and industry uses this equation to calculate MKT.
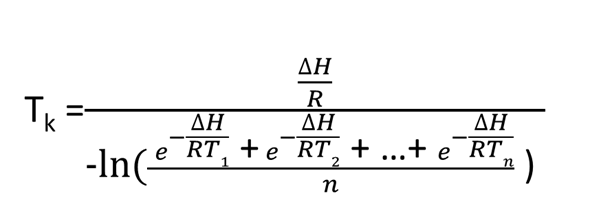

MKT is a weighted nonlinear average that shows the effects of temperature variations over time. However, applying it can only be done in cases where the thermal stability of the product is known and when scientific data is available to support such a claim.
There is much scientific basis for MKT; however, it involves many assumptions and dependencies, e.g., MKT is considered as isothermal storage temperature and is a concept of an integrated time/temperature function. For products whose loss rate constant is related to temperature by the Arrhenius relationship, a single virtual temperature can be determined at which the loss rate is equivalent to that of this changing pattern of temperature. Those assumptions and dependencies, more often than not, are never demonstrated when MKT is brought in to justify temperature excursions.
In USP <659>3 the definition of controlled cold temperature (CCT) is: “The temperature maintained thermostatically between 2°C [36°F] and 8°C [46°F] that allows for an excursion experienced during storage or shipping…” and controlled room temperature (CRT) is defined as: “The temperature maintained thermostatically … between 20°C and 25°C [68°F to 77°F].”
However, thermostatically is not clearly explained.3,4 Most drugs packed in primary packaging, such as a bottle, a vial, a syringe, or a blister, are also packed secondarily in a carton of some sort, individually or grouped, for shipment. Such cartons typically are loaded as a series, and those again are filled as a series in a shipper. These shippers, stacked on a pallet and wrapped with optional corner supports and stretch wrap, are ready for transport. Imagine, in such a composition, there are all kinds of pockets of stationary air, acting as an insulating buffer for the primary packed drug. When a pallet is brought to CRT or CCT and conditioned long enough to be at the required temperature through and through, small spikes of temperature at the outside of the pallet, registered by the mobile temperature logger, may give a very different profile compared to the temperature inside the primary package. The position of the registration device (temperature logger) relative to the drug product is relevant for the registered excursion but not necessarily for the observed temperature variation of the drug product itself.
Advancements In Addressing Shipping Challenges
Developing an ideal vaccine — one that is low-cost, high efficacy, heat stable, freeze tolerant — is not as easy as it may sound. Stability profiles5,6 of proteins/biologics need to be understood because heat-activated enzymes can dramatically alter the degradation rate at a specific temperature. Covalent bonds breaking also can lead to degradation. At sub-zero temperature with solution proteins, agglomeration can occur. Proteins are known to be activated at a body temperature of 37°C, which gives a clear upper limit. Freeze-dried products, on the other hand, when exposed to temperatures as low as -20°C do not show deleterious effects, allowing for extended exposures to temperatures well below 0°C.
Much time has passed since 1971, and even 2001. Now, looking forward, we should consider robust methodologies and smart tools to avoid temperature excursions in stationary and mobile conditions. The construction industry provides solid storage facilities with appropriately sized HVAC, for heating, cooling, and humidifying. The transport industry has proven passive and active temperature-controlled trucks, containers, and other containment systems that can handle large and small transports, bulk shipments and storage, and even single-dose transport and storage if need be, from the first miles out of the manufacturing site to the more difficult last miles to the patient. The packaging industry has many established packaging concepts for vials, syringes, and bottles, to guard them against external exposure of physical natures. Temperature fluctuation is one of the obvious possibilities, but do not overlook physical exposure, both continuous (vibration) and unexpected and sudden movements (shock impact), UV radiation, or humidity. Temperature excursions can also impact closure integrity and compromise the sterility of the product in question.
In general, during storage, handling, and transportation, a drug must be kept under recommended storage conditions, in a particular temperature range according to label instructions, that guarantee the maintenance of its quality and, hence, its safety and efficacy. All possible measures should be taken to avoid exposure of the product to inappropriate temperatures (either too high or too low, as freezing adversely affects adsorbed antigens). The use of temperature loggers or vaccine vial monitors (VVM) can detect drug exposure to temperatures outside the recommended ones.
The Role Of Stability Studies
The stability of a drug, and therefore the proposed shelf life, expiry date, and storage conditions, should be determined based on the results of real-time stability studies. Stability studies should be performed on material representative of the final manufacturing process and final formulation.
Evaluation of the thermal stability of pharmaceuticals is a difficult task. Numerous scientific papers and books address the challenges of evaluating the shelf life of pharmaceuticals. Kinetic investigations of thermal behavior of materials for temperature excursions below or above the range used during data collection are difficult and complex. Such a theoretical and scientific approach is nice in an academic setting but not necessarily a practical representation of the real world.
During the life cycle of the drug — from when it leaves manufacturing and is stored for the first time, through mobile storage, when it may be moved to a central or satellite location and then to a sequence of distribution points or warehouses, to the point where it is delivered at the pharmacy or hospital, and from there to the patient — conditions can be monitored and recorded and samples taken to confirm safety and efficacy.
Data generated in accelerated stability studies may be used, in addition to real-time stability data, to support proposed minimum release specifications, when final product is subject to temperature excursions during handling and shipping.
Stability studies on commercially packaged product should support planned exposures of drugs to temperatures associated with expected temperature excursions, as well as the labeled storage temperature. Such studies include conditions for labeling, packaging, and inspection, as well as shipping of drugs to commercial distributors. Conducting accelerated and long-term stability studies in parallel rather than consecutively saves time.
Shelf-life studies are common for drug products. Temperature cycle tests and, to a lesser extent freeze-thaw studies, should be considered up front as part of the stability study program to be able to address temperature excursion factually instead of academically.
Conclusion
Back in 1971, warehouses, temperature-controlled vehicles, active and passive packaging, and measuring and recording devices were not developed to the standards of today. There may still be reasons to think MKT is an acceptable escape to justify excursions outside the boundaries of comprehensive initial stability, packaging, and transport qualification7 study results. However, the single most prevailing reason we should retire MKT is the fact that we have evolved 50 years. Since MKT’s first introduction, warehouses, temperature-controlled vehicles, active and passive packaging, measuring, and recording devices have matured, have been extensively enhanced, and can be used in real time, anytime, anywhere.
Today, there are no technical limitations or barriers to knowing everything about a shipment or storage facility anywhere on the face of the earth, any time of the year. Designing, planning, executing, and reporting such studies is not a simple task, but that should not cause us to shy away. The safety and efficacy of contemporary drugs, vaccines, and therapies depend on it.
References:
- Journal of Pharmaceutical Sciences – J.Pharm. Sci. Volume 60, issue 6, pages 927-929 (01 June, 1971) – John D. Hayes, - Worldwide Virtual Temperatures for Product Stability Testing
- The Pharmaceutical Journal, 28 July 2001, Volume 267, pages 128-131 – J. Taylor, Recommendations on the control and monitoring of storage and transportation temperatures of medicinal products.
- United States Pharmacopeia USP<659> Packaging and Storage Requirements, USP compendia! monographs
- United States Pharmacopeia USP<1079> Good Storage and Distribution Practices for Drug Products, USP compendia! monographs
- WHO/BS/06.2049 - Final World Health Organisation Guidelines on stability evaluation of vaccines.
- Guide to Control and Monitoring of Storage and Transportation Temperature Conditions for Medicinal Products and Active Substances, Health Products Regulatory Authority HPRA, IA-G011-2 (17 June 2017)
- https://www.pharmaceuticalonline.com/doc/pharmaceutical-transport-validation-where-to-begin-0001
About The Author:
 Tobias Kuners of Koenders is managing consultant at Tob Management, a Netherlands-based consulting firm providing business and technical expertise to the pharmaceutical, biotech, medical device, and FSMP (food for special medical purpose) industries. He has worked with Biogen, Ipsen, Thermo Fisher, Kite Pharma, and Danone, among other companies, gaining hands-on experience in engineering services, equipment qualification, maintenance, supply chain, warehousing, and distribution. He has led design, engineering, and construction of multiple facilities to meet cGMP requirements. He performs audits, identifies gaps, and develops remediation plans, working within organizations to assist with deviation investigations and CAPA implementation and to develop scientific, risk-based solutions. You can contact him at tobias.kunersofkoenders@tobmanagement.nl
Tobias Kuners of Koenders is managing consultant at Tob Management, a Netherlands-based consulting firm providing business and technical expertise to the pharmaceutical, biotech, medical device, and FSMP (food for special medical purpose) industries. He has worked with Biogen, Ipsen, Thermo Fisher, Kite Pharma, and Danone, among other companies, gaining hands-on experience in engineering services, equipment qualification, maintenance, supply chain, warehousing, and distribution. He has led design, engineering, and construction of multiple facilities to meet cGMP requirements. He performs audits, identifies gaps, and develops remediation plans, working within organizations to assist with deviation investigations and CAPA implementation and to develop scientific, risk-based solutions. You can contact him at tobias.kunersofkoenders@tobmanagement.nl
