Pharmaceutical Cleanroom Environmental Contamination Monitoring
Monitor your viable and non-viable particles according to the most recent regulatory requirements. Count, report, document and manage your pharmaceutical environmental monitoring data meeting 21 CFR Part 11 data integrity requirements.
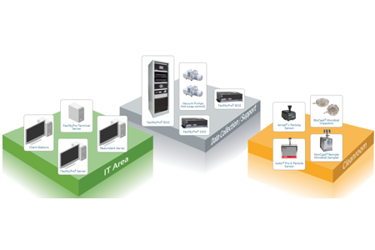
The robust and flexible system architecture from Particle Measuring Systems (PMS) ensures pharmaceutical cleanroom environmental monitoring data is protected and available whenever there is a need for product release and reporting. Solutions for all pharmaceutical cleanroom sizes and classes to fit most budgets.
Detection – Sensors
Viable and non viable samplers from a single manufacturer. Wide variety of analog sensors available including TRH, DAP, Air Flow velocity, etc. Digital inputs and outputs to interface with a selection of operational processes.
Data and Process Management Software
Get actionable insights with the combination of FacilityPro Processors and Pharmaintegrity™ Data Management Software. Designed for GMP and 21 CFR Part 11 compliance, this Environmental Monitoring package includes workload, data and process management, inventory control, and scheduling–all based on identifiable sample locations
