Pharmaceutical Reactors & Crystallizers
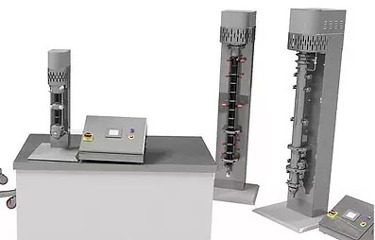
In an effort to move away from conventional batch production and modernizing the global chemical processing industry, continuous manufacturing (CM) is the common vision and desire with the potential to increase efficiency, quality and flexibility in manufacturing.
Dec is one the very few companies able to offer end-to-end CM process solutions, from the incoming raw material handling, to the API synthesis and processing of the compounded drug into the final dosage form, including injectables, and from bench to production scale.
Crystallizers
Ideal for cooling, evaporative and antisolvent crystallization low shear mixing produces little particle attrition with good control over particle size distribution and morphology flexible modes of operation – batch, semi- or fully continuous
Reactors
Fully automated from single vessel batch or continuous stirred tank reactors to multi-vessel continuous or semi-continuous chained stirred tank reactors. Liquid/Liquid, Solid/Liquid and Gas/Liquid capabilities at pressures up to 25 bar and temperatures up to 200 °C. Improved residence time distribution compared to traditional stirred tanks.
Key Features
- Unique chemistry facilitators for gas to liquid hydrogenency
- Liquid, gas liquid and solid liquid reactions and crystallization acting as a catalyst for crystallization
- Highly efficient heat transfer and mixing capabilities
- Excellent crystal size and morphology control
Equipment can be provided with temperature controlled transfer lines to avoid unwanted crystallization or side reactions. Probe adapters available to suit most PAT.
