Reviewing Formulation Technologies For Nanomedicines: How To Select The Right Technology For Your API
By Dr. Andrea Engel, Director of R&D, Evonik Health Care
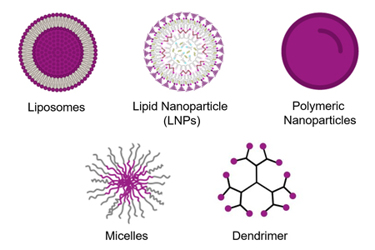
After more than 25 years of commercial use with more than 100 drug products, demand for the formulation development and manufacturing of nanomedicines is surging like never before. More than 1000 nanomedicines are now either in clinical trials or pre-clinical development. From only a handful of journal publications in 2003, publications citing nanomedicines reached almost 5,000 scientific articles in each of the last two years, according to PubMed.
While cancer treatments have long been, and will continue to be, the therapeutic epicenter for nanomedicines, they are also now developed or approved for use across a broad range of other areas such as infectious diseases, central nervous system disorders, cardiovascular diseases and pain management. Most importantly, the approval of the first two mRNA vaccines for COVID-19 in recent months represented a defining industry moment that has brought nanomedicines into the mainstream.
However, the path for any company to translate their prospective nano-formulation into a commercial product is never easy. Companies can expect to encounter many challenges during each stage of a project, including characterization, in vitro evaluation, preclinical efficacy and toxicology studies, GMP compliant scale-up, clinical studies and marketing authorization. In particular, nanomedicine projects will need to overcome hurdles related to formulation complexity, production process limitations, production cost and scalability, as well as market acceptance.
Arguably the most consequential decision that a company will make during their nanomedicine development program will be the selection of the right drug delivery technology. A decision as to which technology is most suitable for an API must be determined by a complex array of factors.
Get unlimited access to:
Enter your credentials below to log in. Not yet a member of Pharmaceutical Online? Subscribe today.
