The Key To Better Visual Inspection Practices
Bridge regulatory gaps in visual inspection and improve product quality by managing defects throughout the product lifecycle. Get a better understanding of how a centralized data repository can help.
Inquest Science has developed a cutting-edge software package that integrates a functional product life cycle approach that will allow companies with visual inspection practices, to review complete product information from raw materials to finished product.
The Identifer Software is designed for evaluating product defect issues along with providing training and guidance on particulate matter control, optimized inspection and 21cfr part 11 compliant electronic particulate and physical container/closure defect tracking systems. Focusing on parenteral, ophthalmic and medical device manufacturing to achieve a robust product lifecycle knowledge and control basis.
Regulatory gaps or assessment points by the FDA, bridged by The Identifier Software:
- Consolidate multi-sourced data electronically from all departments to allow quality assurance management oversight, and to view the full upstream product prior to releasing or rejecting batches.
- Monitor each functional area in the manufacturing train to recognize drift or inconsistencies that may lead to a batch recall.
- Facilitate historical evaluation of inspection or defect testing data to establish realistic control limits. Produce routine SPC or annual product reports for 100% and AQL defect testing, and sub-visible or destructive particulate matter testing.
- Provide a master defect library (photos and descriptions) expected by regulators.
- Maintain a secure (blinded) repository of actual defect standards and test sets. Required for the inspection training and qualification assessments of manual or automated methods.
- Manage qualification data and schedule periodic challenge testing for visual inspectors or vision inspection systems.
- Automate the documentation of manual inspection station setup and system suitability of automated vision systems.
- Provide a simplified mechanism for documenting rejected product characterization to analyze the predominant defects and define action plans for mitigation.
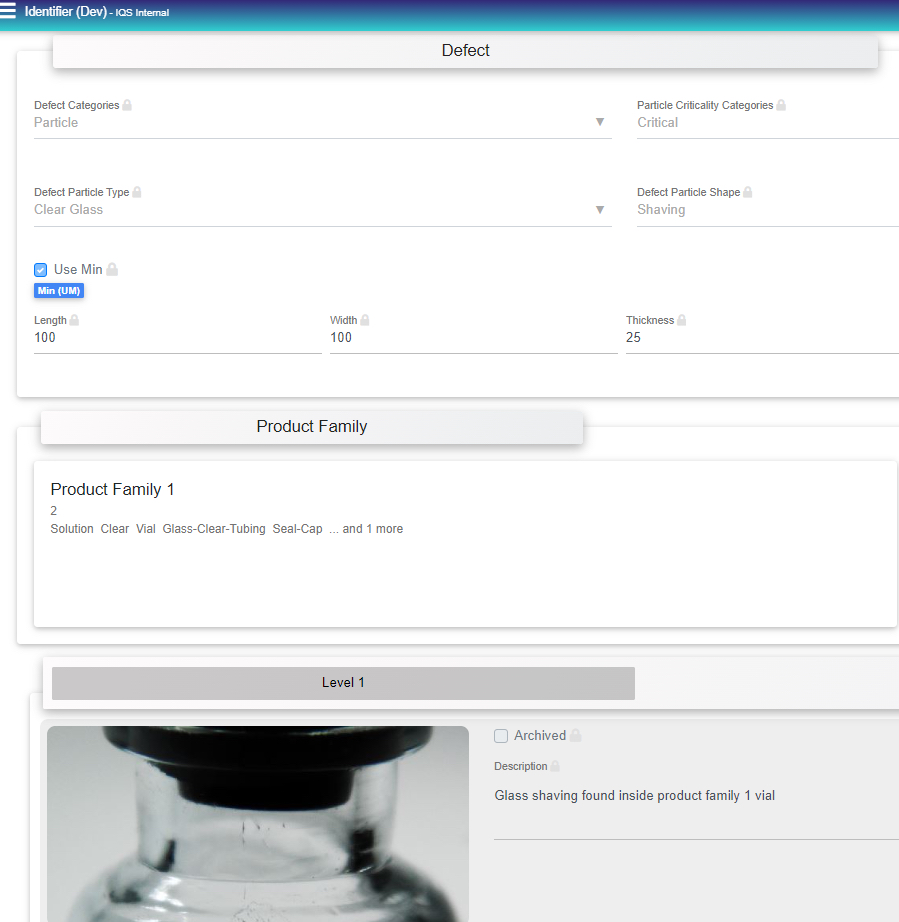
- Capture images and information on particulate matter identification in a standardized three step approach, (levels: 1 (In-situ), 2 (Microscopic), 3 (Spectroscopic).
Advantages of the InQuest Science Identifier Defect Management System over MES
The Identifier Software offers several advantages over using larger Manufacturing Execution Systems (MES) for parenteral visual inspection test set management, training, and qualification of visual inspectors and vision systems.
Specialized Expertise: The Identifier Software is specifically designed by subject matter experts (SMEs) for pharmacopeial parenteral visual inspection, which requires a high level of precision and expertise. It focuses on the unique challenges and extensive requirements of visual inspection in the global pharmaceutical industry, allowing for more specialized and in-depth workflows that the broader MES systems can’t effectively provide.
Enhanced Accuracy: The Identifier Software leverages a digital defect library that contains detailed information about various particulate and physical defects found in parenteral products. This specialized knowledge helps in accurately identifying and classifying defects during the routine inspection process, leading to improved accuracy and reduced false positives or negatives.
Training and Qualification: The Identifier Software’s comprehensive support for training and qualification of visual inspectors and vision systems. It offers targeted training with defect challenge test sets specifically tailored for visual inspection in the pharmaceutical industry, ensuring that inspectors are equipped with the necessary skills and knowledge to meet FDA expectations. The system also facilitates the qualification process, enabling efficient evaluation and documentation of inspector or vision system performance.
Efficient Defect Management: The Identifier Software is a modular system that streamlines the defect management process by providing a centralized platform for recording, tracking, and analyzing visual inspection data. This includes calibration and control of visual inspection test sets, monitoring of training or requalification progress, automated recording of documented testing and qualification of inspection procedures. It offers advanced reporting and analytics capabilities specific to visual inspection, allowing for effective defect trending, root cause analysis, and continuous process improvement.
Integration Flexibility: While larger Manufacturing Execution Systems (MES) may have a broader range of functionalities, they may lack the depth and specialization required for extensive visual inspection processes. The Identifier Software can be seamlessly integrated with MES or other manufacturing information systems, leveraging the strengths of both platforms. This integration flexibility ensures that The Identifier Software can work in harmony with existing manufacturing processes while providing specialized workflows.
Regulatory Compliance: Visual inspection detection test sets and demonstrated inspection qualification are critical aspects of global pharmaceutical manufacturing, and regulatory agencies have specific requirements regarding defect management and inspection processes. The Identifier Software is designed to meet these regulatory standards, providing documentation and audit trails to support compliance efforts aligned with USP-790, USP-1790 guidance and world-wide expectations.
The Foundational Modules and features of the Identifier Software
Scheduling Module- Eye exam scheduling module. Schedules and tracks all inspectors’ past, current, and future eye exam statuses
Finished Product Library Module- Defines all defects and facilitates training. Digitally provides a master description, images and criticality categories for all defects intrinsic, extrinsic and inherent to incoming product materials or finished products. Defect Library entries are created and stored for training and test kit purposes.

Defect Standards Repository Module – Challenge/Test Kit Data Management- Provides an inventory of physical units that represent specific defects. Full blinding of the test set units to eliminate bias and maintain data integrity. Expiration dates and PoDs automatically tracked.
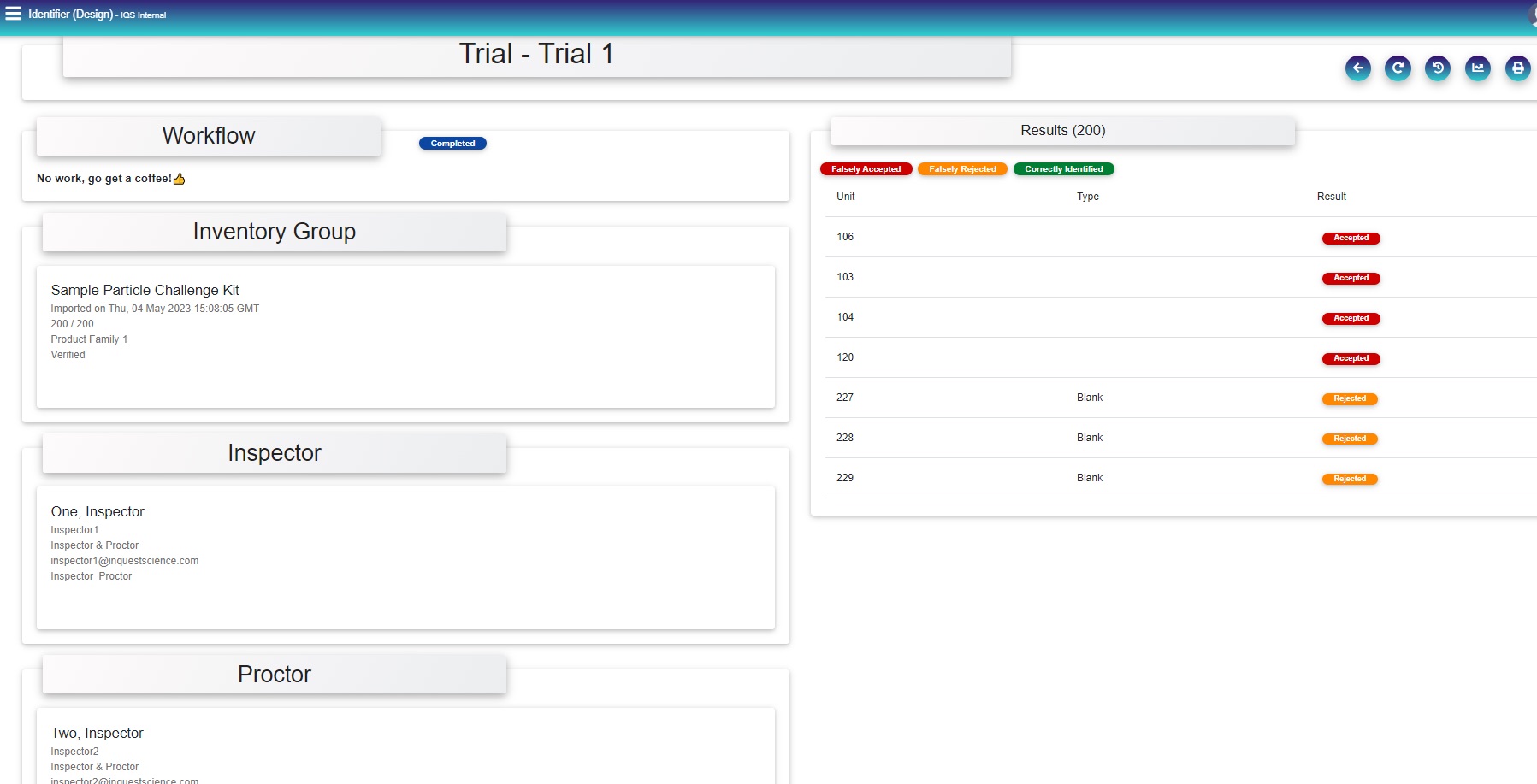
Calibration & Knapp Module- Calibration workflows of inspectors for Test Kits. System automatically generates probability of detection for each unit, along with the automatic tracking of unit expiration dates. Knapp Zone data generated and stored.
Training & Qualification Module- Inspector and Automated Vision Inspection Machine Studies: Calibrated Test Sets used to train inspectors and qualify both manual and automated visual inspection *Includes Daily System Challenges for AVI Machines
Reporting & Audits- Software includes a full audit trail for every action made in each module. System generated audit trail reports can be exported
Additional modules in development - 100% Inspection & Reject Characterization
Digital systems can facilitate compliance with FDA or global pharmaceutical industry standards and regulatory requirements. They also simplify the auditing process by providing a clear audit trail and easy access to historical data. The Identifier software has specialized user-oriented work flows for organizational efficiency. The integrated modules connect the defect library with defect standards management, test assignments to inspector training and manual inspection or vision system qualification to acceptance criteria. In addition, the system facilitates the documentation of particle detection threshold testing or Knapp zone comparison. Future modules such as 100% in process inspection and AQL inspection will integrate seamlessly with existing modules.
The Identifier software eliminates the time used for manual paper-based raw data recording and reentry of data to spreadsheets for calculations. Replacing filing and physical storage of documentation. A digital database allows for quicker data entry, retrieval, and analysis compared to manual methods. This can lead to reduced administrative workload and faster decision-making.
Human errors associated with manual data entry, calculation mistakes, and data duplication or manipulation are minimized, The Identifier software supports data integrity. Providing real-time access to information, enabling authorized managers to access relevant data from anywhere at any time.
