Expert data management system for the 21st century in particulate & defect tracking.
Inquest Science has developed a cutting-edge defect management software package that integrates a functional product life cycle approach. Allowing companies with visual inspection practices to review complete product information from raw materials to finished product.
The Identifier Software is designed for evaluating product defect issues along with providing training and guidance on particulate matter control, optimized inspection and 21cfr part 11 compliant electronic particulate and physical container/closure defect tracking systems. Focusing on parenteral, ophthalmic and medical device manufacturing to achieve a robust product lifecycle knowledge and control basis.
Be a leader in the eyes of the FDA with InQuest Science’s defect management software. Take the next steps into the future of Pharma 4.0.
- Are you still working with paper and Excel spreadsheets?
- Are you barcode blinding your test kits?
- Is your defect library stored in a digital database?
![]()
Did you know The Identifier software will:
- Eliminate the time used for manual paper-based raw data recording and re-entry of data to spreadsheets for calculations.
- Facilitate compliance with FDA or global pharmaceutical industry standards and regulatory requirements.
- Replace filing and physical storage of documentation. A digital database allows for quicker data entry, retrieval, and analysis compared to manual methods.
- Eliminate human errors associated with manual data entry, calculation mistakes, data duplication and manipulation.
FEATURED VIDEOS

Why Choose The Identifier Software?
Unlock Pharma 4.0 for injectable drug inspection. Learn about a system that transforms data management, offering immediate access and global regulatory compliance.
-
Transferring visual inspection data from paper to digital is essential for Pharma 4.0 compliance. Learn practical strategies to eliminate errors, automate data tracking, and enhance quality assurance.
-
Digitizing your visual inspection processes is key to unlocking efficiency and ensuring compliance with global regulatory standards. Learn how to transition from paper-based systems to a digital database.
-
Discover how expert database systems for inspection defect management and control help cell and gene therapies meet the requirements of USP-790 to assure they are essentially free from particulate matter.
-
Discover how a truly blind system eliminates bias from the human inspection process, improving data accuracy and validation of automated inspection systems.
-
Many companies don't formally track the training data from their new visual inspectors, missing out on valuable insights into their progress and readiness for qualification.
-
A biased inspection process can create a false baseline, negatively impacting the ability to accurately calibrate and validate automated inspection systems.
CONTACT INFORMATION
InQuest Science
617 Stokes Rd STE 4334
Medford, NJ 08055
UNITED STATES
Phone: 855-522-2100
SOLUTIONS
-
Move your visual inspection processes into the Pharma 4.0 era with a digital system that provides data integrity and full audit trails to aid in compliance.
-
Bridge regulatory gaps in visual inspection and improve product quality by managing defects throughout the product lifecycle. Get a better understanding of how a centralized data repository can help.
FEATURED INSIGHTS
-
Safeguarding Quality In Parenteral Drug Manufacturing
Find out how vision inspection software helps injectable drug manufacturers meet USP <790> standards by improving defect detection, ensuring data integrity, and eliminating paper-based risks.
-
Vision Inspection Software: The Missing Link In Digital Transformation
AI and MES can’t meet pharma’s visual inspection demands. Discover how purpose-built software ensures defect detection, compliance, and paperless quality for safer, more efficient operations.
-
Why A Dynamic, Digital Defect Library Is Now A Regulatory Expectation
Maintaining a dynamic, digital defect library is essential for modern sterile manufacturing. Learn how this approach helps ensure consistency in batch disposition and supports regulatory compliance.
-
Test Kit Management: The Hidden Weak Point In Your Visual Inspection Program
A proper test kit management program is no longer a “nice-to-have”—it’s a compliance necessity. Without a structured system, you risk inspector drift, failed audits, and errors in batch release.
-
Why Is It Important To Have Properly Trained Visual Inspectors?
Properly trained visual inspectors are crucial for identifying defects in parenteral drugs, safeguarding patient safety and product quality.
-
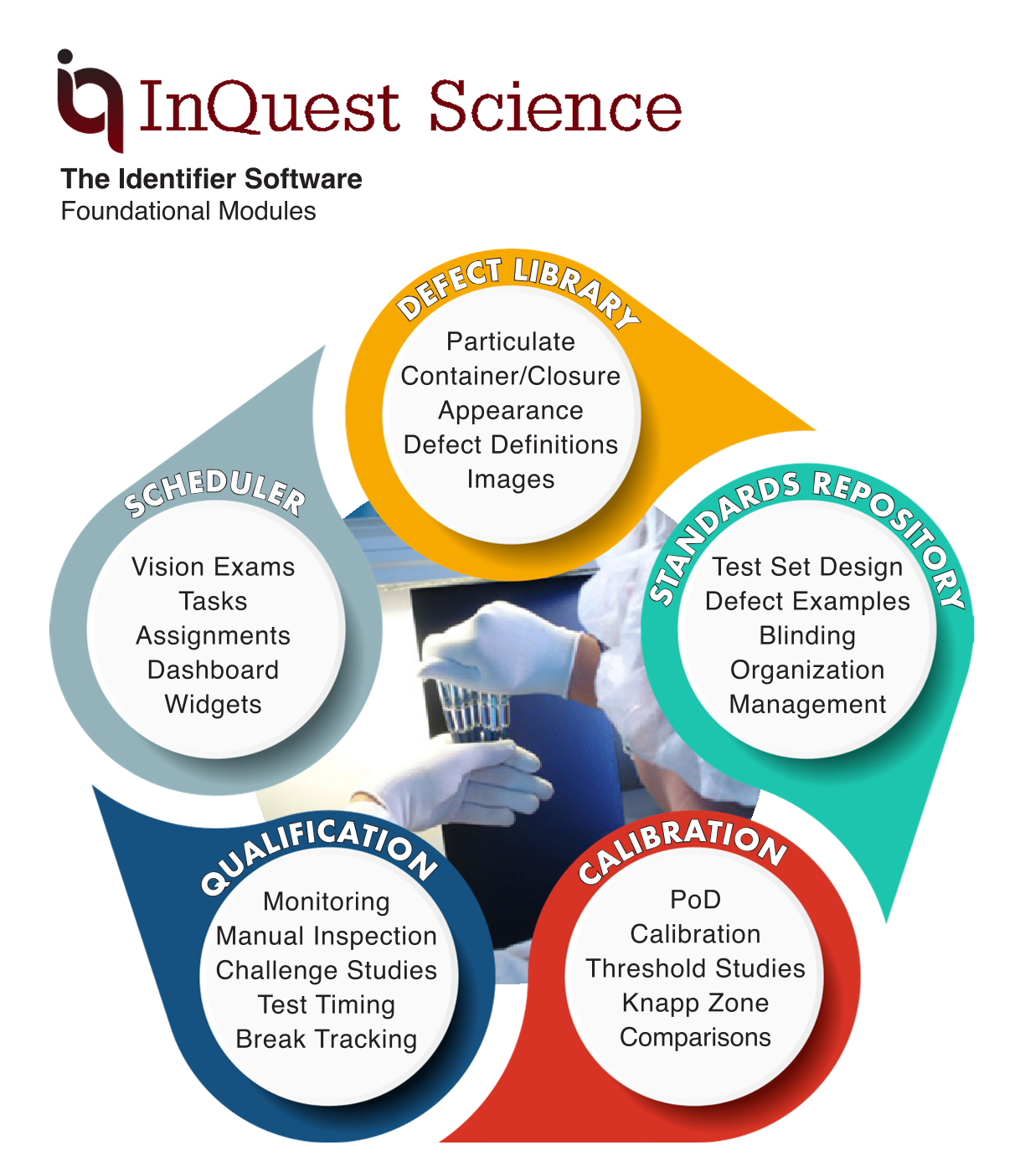
The Identifier Software: Foundational Modules
Be a leader in the eyes of the FDA with InQuest Science’s defect management software. Take the next steps into the future of Pharma 4.0.
-
Building A High-Fidelity Manual Baseline For Tomorrow's Automated Inspection
Future-proof your pharma manufacturing. Discover why manual visual inspection remains foundational and how advanced software can build a high-fidelity baseline for automation success.
-
6 Things To Consider During Visual Inspection Operations
Even with automation, manual visual inspection remains vital in sterile drug manufacturing. Discover six advanced considerations for refining your operations, ensuring compliance, and minimizing costs.
-
How Software-Enabled Manual Visual Inspection Can Boost Your ROI
Unlock hidden cost savings in pharma manufacturing. Learn how software-enabled manual visual inspection enhances efficiency, data integrity, and regulatory readiness without full automation's capital expense.