What Patients Say Matters: How AI Analyzes Sentiment to Improve Generic Drug Oversight
By Ajaz S. Hussain, Ph.D., independent pharmaceutical regulatory science expert

The expectancy effect, encompassing placebo and nocebo responses, is pivotal in how patients perceive and respond to medications. In clinical trials, placebo controls isolate pharmacological efficacy. Still, in real-world settings, patient expectations are shaped by trust in healthcare providers, regulatory bodies like the U.S. FDA, and quality assurance systems. This trust underpins the assumption that safety, efficacy, and therapeutic equivalence established during FDA approval persist throughout a drug’s lifecycle.
For generic drugs, however, this assumption faces challenges. Despite being approved as therapeutic equivalents to reference-listed drugs (RLDs), generics often differ in appearance, color, shape, or packaging due to trademark restrictions. When trust diminishes, as it did during 2012-2014 when a cluster of FDA warning letters mushroomed,1 research indicates that such differences can impact adherence: a change in pill color increases non-adherence risk by 34% (adjusted odds ratio 1.34; 95% CI, 1.12–1.59), and a change in shape by 66% (adjusted odds ratio 1.66; 95% CI, 1.43–1.94).2 In response, the FDA issued a guidance in 2014, updated in 2022, on the importance of sameness in shape and size, omitting color.3
Why was color omitted? Color is often a trademark, a perpetual brand identity, unlike the expiration of intellectual property. As seen in the “purple pill” case, a court blocked Dr. Reddy’s generic Nexium due to its similarity to AstraZeneca’s branded version.4 Such legacy constraints imposed on generic drug development often become a regulatory blind spot.5
Sentiment analysis, a natural language processing (NLP) technique,6 provides a new way for the FDA and the industry to be sensitive to patient needs and improve communication and education. By analyzing patient-generated content, researchers can reveal how perceptions shape drug experiences beyond clinical trial data. This article explores the potential of sentiment analysis through a case study on generic metoprolol succinate ER tablet, a generic beta-blocker that has repeatedly faced recalls. It emphasizes the importance of appreciating patient sentiment and how it relates to the erosion of quality assurance. It also explains why minimizing external failures is crucial by achieving a Six Sigma level of error reduction — by breaking the 2-3 sigma barrier — and requires effective corrective and preventive action (CAPA) and a commitment to continual improvement.7
Methodology
Approximately 79 public comments in response to The People’s Pharmacy article titled “Another Metoprolol Recall Reveals the Dark Side of Generic Drugs”8 were analyzed by the author using three AI models: Grok (xAI), DeepSeek (OpenBMB), and ChatGPT-4 (OpenAI). These models were prompted to classify sentiments as positive, negative, or neutral and to identify emotional tones and themes within the comments. Employing multiple models ensured triangulation, which minimized bias and enhanced reliability. The comments, collected from posts made between 2014 and 2019, reflect patients' reactions to recalls and concerns about perceived quality.
Results
The sentiment analysis revealed a predominantly negative response:
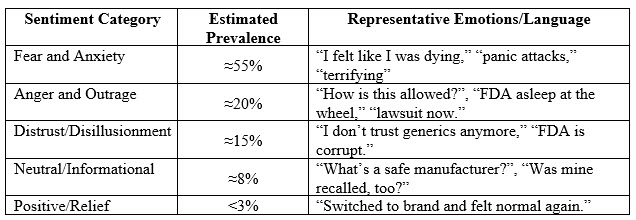
Key Themes
- Therapeutic Disruption: Patients reported physiological issues — e.g., arrhythmias, blood pressure spikes, and hospitalizations — after switching generics, challenging therapeutic equivalence claims.
- Erosion of Trust: Recurring distrust in the FDA, pharmacies, and foreign manufacturers (e.g., “Indian pills are poison”) signaled a loss of faith in regulatory oversight.
- Adherence Breakdown: Changes in appearance and quality fears led to non-adherence, with some patients discontinuing treatment (as confirmed later3) or incurring costs to switch to brand-name drugs.
Model-specific insights varied slightly: Grok emphasized lexical cues (e.g., “terrifying”), ChatGPT focused on emotional intensity (e.g., “worthless”), and DeepSeek highlighted distrust in foreign manufacturing. Despite these nuances, all models confirmed negative sentiments dominated (85%–90%), with fear/anxiety most prevalent.
What Do These Findings Mean And What Can We Do?
The findings highlight the impact of the expectancy effect on the effectiveness of drugs in real-world scenarios. Negative feelings, particularly fear and distrust, can intensify nocebo effects,9 which in turn diminish the perceived effectiveness of FDA-approved generics, such as metoprolol succinate ER tablets. External (exposed to the public) issues like quality failures, product recalls, and warning letters significantly influence patient experiences. This indicates that quality assurance should go beyond simply proving bioequivalence; it must also encompass the overall evidence, including current good manufacturing practices (cGMP), continuous process verification, and an understanding of the psychological and perceptual factors that shape how we communicate about quality failures. Companies can document such information during their annual product reviews and in their annual reports to the FDA.
Sentiment analysis offers a scalable, proactive tool for pharmaceutical quality assurance. It can guide interventions, e.g., improved manufacturing oversight or patient education, to enhance adherence and outcomes by capturing early signals of therapeutic disruption and institutional distrust. For stakeholders:
- Pharma Companies: Sentiment data from consumer feedback on social media and formal complaints can help identify quality issues, allowing for prioritized corrective actions and continual improvements in formulation or process, and documented as part of annual product review (and reported to the FDA in annual reports).
- FDA: Integrating post-marketing surveillance and annual reports can enhance adverse event reporting and assessment and help address some of the gaps in regulatory oversight. For instance, it highlights the importance of annual reports to ensure ongoing assurance of therapeutic equivalence.
- Healthcare Providers: Insights into patient fears allow tailored support to mitigate negative expectations.
This case study illustrates a point; future research could expand data sets and refine AI models to distinguish nuanced sentiments better.
Conclusion
The metoprolol case demonstrates how sentiment analysis can uncover critical gaps in pharmaceutical quality assurance and provide a pathway for valuing patient experience. With emerging evidence linking Indian-manufactured generics to higher rates of severe adverse events,10 the urgency to modernize post-market surveillance has never been greater. What patients say matters, and perception often shapes outcomes. Sentiment analysis offers a scalable tool to surface early safety signals, enhance transparency, and restore public trust. By incorporating patient sentiment into regulatory evaluation, therapeutic equivalence can be redefined — not merely as a technical designation, but as a standard genuinely experienced and trusted by those it is meant to serve.
References
- Confronting Illusions of Quality in Indian Generics Manufacturing, By Bowman Cox, PINK Sheets, 28 Mar 2014 https://insights.citeline.com/PS000821/Confronting-Illusions-of-Quality-in-Indian-Generics-Manufacturing/
- Kesselheim, AS, Bykov, K, Avorn, J et al. Burden of Changes in Pill Appearance for Patients Receiving Generic Cardiovascular Medications After Myocardial Infarction: Cohort and Nested Case–Control Studies. Ann Intern Med. 2014;161:96-103. https://www.acpjournals.org/doi/10.7326/M13-2381
- FDA GUIDANCE FOR INDUSTRY: Size, Shape, and Other Physical Attributes of Generic Tablets and Capsules. Revised October 2022. https://www.fda.gov/media/161902/download
- No ‘purple pill’ for Dr. Reddy’s—court sides with AZ, blocks Nexium generic over coloration. BIOPHARMDRIVE. Published Nov. 10, 2015, By Sy Mukherjee. https://www.biopharmadive.com/news/no-purple-pill-for-dr-reddyscourt-sides-with-az-blocks-nexium-generic/408952/
- Hussain, A.S. Addressing Blind Spots In Assuring Therapeutic Equivalence. Pharmaceutical Online, January 14, 2025. https://www.pharmaceuticalonline.com/doc/addressing-blind-spots-in-assuring-therapeutic-equivalence-0001
- Jim, J. R., Talukder, M. A. R., Malakar, P., Kabir, Md. M., Nur, K., & Mridha, M. F. (2024). Recent advancements and challenges of NLP-based sentiment analysis: A state-of-the-art review. Natural Language Processing Journal. https://doi.org/10.1016/j.nlp.2024.100059
- Hussain, A. S. How To Break The Pharmaceutical 2-3 Sigma Barrier (Like Amgen). Pharmaceutical Online, September 18, 2017. https://www.pharmaceuticalonline.com/doc/how-to-break-the-pharmaceutical-sigma-barrier-like-amgen-0001
- Another Metoprolol Recall Reveals the Dark Side of Generic Drugs. By Joe Graedon. The People's Perspective on Medicine, The People's Pharmacy. February 28, 2017. https://www.peoplespharmacy.com/articles/another-metoprolol-recall-reveals-the-dark-side-of-generic-drugs
- Hussain, A.S., Gurvich, V.J. & Morris, K. Pharmaceutical “New Prior Knowledge”: Twenty-First Century Assurance of Therapeutic Equivalence. AAPS PharmSciTech 20, 140 (2019). https://doi.org/10.1208/s12249-019-1347-6
- Noh, I. J., Gray, J., Ball, G., Wright, Z., & Park, H. (2025). Are All Generic Drugs Created Equal? An Empirical Analysis of Generic Drug Manufacturing Location and Serious Drug Adverse Events. Production and Operations Management, 0(0). https://doi.org/10.1177/10591478251319691
About The Author:
 Ajaz S. Hussain, Ph.D., is a pharmaceutical quality and regulatory science expert with decades of experience across academia, industry, and government. As a former deputy director of the FDA's Office of Pharmaceutical Science, he was pivotal in pioneering initiatives like the process analytical technology (PAT) framework and the Pharmaceutical Quality for the 21st Century Initiative. Hussain also served as president of the National Institute for Pharmaceutical Technology and Education (NIPTE), advancing pharmaceutical technology and regulatory science. Now an independent consultant, he empowers organizations to integrate design thinking with a systems approach to advance innovative technologies that hold high potential for enhancing patient care and operational excellence.
Ajaz S. Hussain, Ph.D., is a pharmaceutical quality and regulatory science expert with decades of experience across academia, industry, and government. As a former deputy director of the FDA's Office of Pharmaceutical Science, he was pivotal in pioneering initiatives like the process analytical technology (PAT) framework and the Pharmaceutical Quality for the 21st Century Initiative. Hussain also served as president of the National Institute for Pharmaceutical Technology and Education (NIPTE), advancing pharmaceutical technology and regulatory science. Now an independent consultant, he empowers organizations to integrate design thinking with a systems approach to advance innovative technologies that hold high potential for enhancing patient care and operational excellence.
