
INSPECTION
Why Add Water Activity To The Stability Test Protocol
In this video we describe a framework on how by adding water activity measurements to stability protocols will achieve to improve the quality program.

Protecting Oxygen Sensitive Formulations Throughout Product Life Cycle
This webinar reviews how oxygen levels in finished parenteral drug containers can be determined and controlled throughout the product life cycle by using laser-based headspace analysis.

A Data-Driven Approach To Container Closure Integrity
Gain confidence in your container closure system selection by utilizing a stepwise and data-driven approach to predicting and verifying container closure integrity.

The Container Closure Integrity Requirements In The Revised EU Annex 1
Learn about the advantages and disadvantages of CCI testing methods, how the revised EU Annex 1 may impact your strategy for ensuring CCI of sterile pharmaceutical products, and more.

Early-Stage Considerations For The Selection Of Pre-Fillable Syringes When Developing A Vaccine
Get an overview of the strategy to speed up the migration from a vial to a pre-fillable syringe. Explore the main considerations in meeting and overcoming PFS-drug challenges when developing a vaccine.

A Rapid Approach For Moisture Determination Of Lyophilized Product
Explore the limitations of traditional moisture determination techniques, an innovative approach using laser-based headspace analysis, and real-world case studies using this non-destructive method.
Mitigating Ultracold Storage And Transport Risk With CCIT
Dr. Derek Duncan, Director of Product Lines, Lighthouse Instruments, and Brandon Zurawlow, Chief Scientific Officer, CS Analytical, discuss a program for generating packaging data for deep cold storage products.
Survival Kit For Quality Regulations: A Closer Look At Lyo Packaging
Explore primary packaging developed following well-established ICH principles for sustainable compliance and vial container closure systems for product development and commercial manufacturing needs.
Rapid Non-Destructive Product Moisture Determination In Stability Studies
Examine whether Karl-Fischer (KF) titration can be replaced by rapid, non-destructive lyo product moisture determination method.

Selecting Container Closure Systems With Confidence: Lyophilization
Experts take you through the entire selection process for primary packaging components for lyophilization and provide aspects to consider for all relevant attributes.
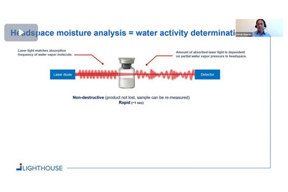
Replacing Total Moisture Measurements By Water Activity Determination
This short video explains the difference between total moisture content, headspace moisture, and the relation to water activity.
Container Closure Integrity Of Product Requiring Deep Cold Storage
Gain insight into the risk to container closure integrity (CCI) during deep cold storage and identify appropriate analytical tools, to generate robust data to characterize and mitigate the risk to CCI.

A Science-Based Approach For Ensuring Container Closure Integrity Of Sterile Vials
Recent regulatory guidance has triggered changes in industry best practices in the area of container closure integrity (CCI) testing. A more science-based holistic approach that includes robust design & qualification of the process and the implementation of appropriat...

USP <922> Water Activity Has Opened A Door For OSD Manufacturers
Learn more about USP <922> Water Activity and why using Karl-Fischer titration to measure total water content is no longer adequate to fully understand the stability of oral solid dosage products.

A Practical Road Map For Compliance To EU GMP Annex 1
Learn how to interpret the Annex 1 container closure requirements, develop deterministic analytical methods for CCIT, and design packaging studies that generate robust data demonstrating good CCI.
