
QUALITY CONTROL

An Efficient And Reliable Way To Digitalize Your Sterility And QC Testing Data
Watch to learn about the M-Trace™ Electronic Test Record Software, your digital QC companion for trusted and efficient data recording that automizes and digitalizes your sterility and QC testing data.

The Route To Faster Microbial Quality Control
Join product and application experts Anne-Grit Klees, Carine Krebs, and Esther Welterlin to discover the benefits of using a new rapid microbial detection platform.

Making Sense Of Analytical Standards In Pharma QC
Reference materials are a critical component of the analytical testing workflow. Learn about metrological traceability, the hierarchy of reference materials, fit-for-purpose selection considerations, and more.
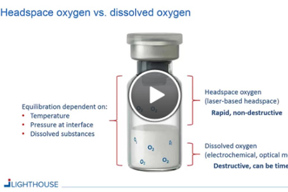
Determining And Controlling Oxygen Levels In Sensitive Formulations
This webinar will review how oxygen levels in finished parenteral drug containers can be determined and controlled throughout the product life cycle by using laser-based headspace analysis.
Mitigating Ultracold Storage And Transport Risk With CCIT
Dr. Derek Duncan, Director of Product Lines, Lighthouse Instruments, and Brandon Zurawlow, Chief Scientific Officer, CS Analytical, discuss a program for generating packaging data for deep cold storage products.

Maximizing Safety In The Daily Handling Of Hazardous Goods In The Lab
Understand the role of safety in the lab as well as the challenges of handling hazardous goods and how you can overcome them.
Rapid Microbial Detection System For Earlier Results
Learn about the Milliflex® Quantum system, a fluorescence-based microbial detection system that enables you to read results days earlier than traditional methods.

Webinar On-Demand: Substitution Of Hazardous Substances
Substitution is the essential starting point in every risk assessment. Learn how to conduct a legally compliant substitution check using real substances as examples.

Selecting Container Closure Components: Extractables And Leachables
Primary packaging components require a thorough assessment to ensure safety and efficacy. Plan ahead and start your assessment early to mitigate your risks, delays, and out-of-pocket costs.

Using Positive Controls In Container Closure Integrity Studies
This webinar describes the use of positive controls as an important element of CCI studies designed to validate packaging components for CCI or to qualify processes for producing good CCI.

Data Integrity In The Quality Control Lab: No Pen, No Pain
Data integrity in pharmaceutical QC labs is critical for product safety. This webinar explores digital solutions to improve traceability, reduce paperwork, and enhance workflows.

The Future Of Robotics And Automation In QC In Microbial Testing Labs
Automation based on the use of robotics and digital tools will transform the QC process in microbial testing labs to meet the productivity, data integrity, and reliability requirements of Pharma 4.0.

Microbial Sampling Of Compressed Gas Has Never Been So Simple And Safe
Discover the key features of an innovative microbial compressed gas air sampler, designed for the user's peace of mind, ease of use, maximum safety, and full regulatory compliance.

Workflow For Rapid Sterility Testing
Learn about the workflow for an automated solution for the rapid detection, imaging, and quantification of viable microbial contaminants in filterable samples at various stages of the manufacturing process.

Compressed Gas Risk Assessment: A Significant Step In Your CCS
Discover the importance of compressed gas monitoring in ensuring product quality and compliance with EU GMP standards.

Expanding Capabilities In QC Analyses With Advanced LC Detection
Today's quality control (QC) labs need instruments that can accurately take on a wide range of tasks. Discover a system that allows QC labs to enhance their capabilities and achieve various goals.

Determining CCI During Deep Cold Storage
Deep cold storage, at dry ice (-80°C) or even cryogenic (-196 °C) temperatures, poses a significant challenge to packaging components and can result in CCI failures.
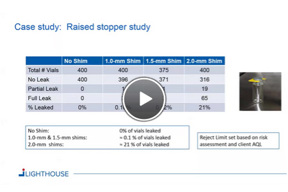
CCI Testing And Revisions To EU Annex 1
The new language in EU Annex 1 will likely have a significant impact on your CCI testing practices. In this webinar CCI testing strategies and the proposed revisions to EU Annex 1 are discussed.

Bioburden And EU GMP Annex 1: Risk Assessment, Reduction, And Testing
Experts on regulation, filtration, sampling, and quality control discuss risk assessment and filtration-based strategies to reduce bioburden.
