FEATURED ARTICLES
-
All You Need To Know About Contamination Control Strategies, Part 1
In the first of this two-part series, microbiology and contamination control specialists Vanessa Figueroa, Rebecca Brewer, and Greg Gibb, Ph.D., discuss best practices in developing contamination control strategies through the lens of the newly formalized provisions in the EU GMP Annex I, Manufacture of Sterile Medicinal Products.
WHITE PAPERS & CASE STUDIES
-
Patient Adherence with A Novel Dosage Form
A sprinkle formulation helps patients with tremors or dysphagia take medication more easily, improving adherence, comfort, and care through a novel, FDA-approved oral granule format.
-
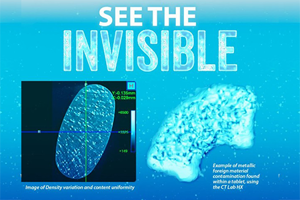
A Powerful Non-Destructive Tool For Tablet Characterization
Discover how X-ray micro-CT provides vital, non-destructive analysis of a tablet’s internal 3D physical structure. This structural insight is essential for troubleshooting defects and confirming performance.
-
Optimizing DPI Production: Filling Technologies For Precision And Performance
Developing effective dry powder inhalers requires expertise across multiple disciplines, from particle engineering and device technology to manufacturing science and regulatory knowledge.
-
How To Avoid Sticking And Picking In The Tableting Industry
Review strategies to mitigate sticking and picking risks in the tablet industry, and learn how you can foster improved tablet production processes, ensuring higher quality and consistency.
-
Overload Setting – Tricks And Techniques
Learn how a partner company that is committed to assisting manufacturers with calibration and optimal set-point establishment can serve as a valuable resource in achieving optimal tablet production.
-
Seizing Market Share Pre-Patent Expiry: The Evolution Of A CDMO Partnership In Navigating A Paragraph IV ANDA Submission
The dynamic pharmaceutical landscape demands agility and strategic foresight. Explore how leveraging a key partnership helped to overcome a looming patent expiry for a second-generation acne medication.