ARTICLES BY MATTHEW PILLAR
-
Antibodies 2025: Venerable & Naked Or Complex & Multi-Specific?12/19/2024
Antibodies are the biotherapeutic bulwark, the steadfast stalwart in not just oncology, but infectious and chronic inflammatory diseases as well. We consulted with a variety of antibody developers for insight into the challenges and opportunities antibody modalities face in the coming year and beyond.
-
The ADC Market Is Ripening For Disruption8/21/2024
Where there is therapeutic demand, the industry will build capacity. But with ADCs, cytotoxic payloads and a fragmented contract manufacturing landscape make the path to clinical and commercial supply dauntingly complicated. Here's how the ADC manufacturing market is shaping up, and how it might ultimately shake out.
-
Are You Taking The HPCs In Your ADCs Seriously?8/16/2024
The wave of ADC (antibody-drug conjugate) development and manufacturing activity we’re currently witnessing, and importantly, the HPC (high-potency compounds) comprising the payloads in these therapies, demand thorough assessment of development and manufacturing facility design.
-
BPSA's Single-Use Summit Celebrates Its "Stainless" Anniversary7/29/2024
The Bio Process Systems Alliance (BPSA) is devoted to the advance of single-use technologies in biopharmaceutical manufacturing. At its 11th International Single-Use Summit, the explosive growth in single-use was on full display, and the sourcing, safety, sustainability, and application discussions on the agenda were comprehensive.
-
Scale And Sustainability In Single-Use Systems6/13/2024
I had the opportunity to cover a lot of ground on considerations for single-use technologies in biopharmaceutical manufacturing with Mark Petrich, Ph.D. when he joined me as a guest expert on the Bioprocess Online Live event Single Use Technologies For Bioprocessing: An Essential Update. Here are some of his insights on single-use scale and lifecycle management.
-
Where's The Case For Generative AI In Biopharmaceutical Manufacturing?6/12/2024
The early use cases for AI in the biopharmaceutical industry—at least, the early public use cases—have largely come from R&D, and more specifically, target identification and molecular design. Where are the use cases in biologics manufacturing, supply chain management, QMS, and operations, and what’s holding us back?
-
Single Use In Biopharma: Beyond Savings & Sustainability4/17/2024
SUT continues to trend in biopharmaceutical applications, driven largely by environmental and economic considerations. But there’s a lot more to the SUT story, including supply chain and standardization advantages. We dove headlong into those issues and more with independent SUT expert Paul Priebe and Krystal Biotech VP of Technical Operations Mark Petrich.
-
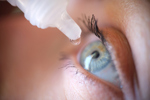
Eying Up The Era Of Topical Biologics3/19/2024
Claris Bio’s phase 1/2 clinical trial in patients with Stage 2 or 3 Neurotrophic Keratitis (NK) is breaking new ground in the development of topically administered biologic therapies.
-
Debra Weiss, RN: Big Impact In Small, Non-Profit Bio3/8/2024
The career of Debra Weiss, RN, MSN, and COO at the biopharma Gates Medical Research Institute (Gates MRI), offers a case study in creating success through service-oriented leadership.
-
Antibody-Drug Conjugates: Increasing Investment In R&D And Partnership2/1/2023
DeciBio’s Antibody-Drug Conjugates 2023 Industry Survey points to progress in ADC development. Respondents anticipate lower regulatory, financial, and R&D barriers as the technology matures. Senior Life Science Expert Joe Daccache, Ph.D. gave us an insider's look at the numbers.
-
The Coming Wave Of Radio(bio)pharmaceuticals6/13/2022
Convergent Therapeutics is bullish on the combination of radioisotopes and antibodies to direct radiation directly to cancer cells. As enabling technologies improve on previous failures, CEO, CMO, and co-founder Dr. Philip Kantoff is projecting a new wave of activity in the space among biopharmas big and small.
-
Computational Science: Disruption In Biopharma Discovery3/12/2021
NeuBase Therapeutics CEO Dr. Dietrich Stephan discusses the important role computational drug discovery plays as we emerge from what he characterizes as “a decades-long, multi-billion-dollar chemical engineering escapade with a very low success rate to find one drug.”
-
Are Dry Powder Biologics Ready For Primetime?9/21/2020
A new approach to freezing biopharmaceuticals into fine, dry powders holds promise for flexible therapeutic form factors, efficacy improvements, and supply and distribution chain efficiencies. It's a particularly timely development in the age of COVID-19.
-
The Compounded Risk Of Early-Stage Biopharma Licensing Deals6/25/2020
For emerging biopharma firms, licensing options can equal a boon to the business when cash runways are short and financing options run thin. But, there’s a catch. Most licensed candidates fail. And, according to a new paper, rushed licensing deals on the heels of phase 3 clinical failures only compound the disappointment.
-
Mechanistic Action: Genesis Of A Biologics Pipeline?6/17/2020
Cue Biopharma President & CSO Anish Suri, Ph.D. tells us why a partnership with The University Of Oxford to visualize the mechanistic action of molecules at the immunological synapse could provide insights that guide future drug discovery and development strategy.