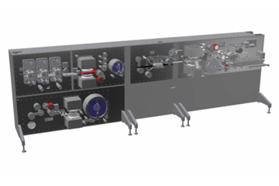
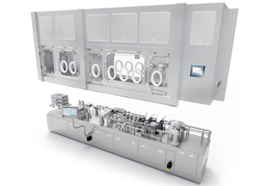
3P innovation is the UK’s leading supplier of aseptic fill-finish solutions, supporting the pharmaceutical and biotechnology sectors. We deliver precision-engineered aseptic fill-finish solutions for pharmaceutical manufacturers and CDMOs working with high-value medicines. Our modular, option-driven platforms can be customised precisely to your requirements, with sophisticated integrated technology that differentiates our solutions in the market.
We help customers scale efficiently, maintain accuracy, and ensure compliance throughout product development and manufacturing. With premium engineering quality and exceptional adaptability, we specialise in intricate applications where standard equipment falls short. We combine the technical sophistication of larger competitors with the responsiveness and attention to detail that truly sets us apart. Our proof-of-principle methodology significantly mitigates innovation risk, while dedicated support ensures prompt resolution of issues. Through this distinctive approach, manufacturers innovate with confidence, accelerate time-to-market, and achieve manufacturing excellence even in the most stringent environments.
CONTACT INFORMATION
3P innovation
Tournament Fields, Bosworth Avenue
Warwickshire, CV34 6UQ
UNITED KINGDOM
Phone: +44 (0) 1926 408933
Contact: David Johnson, Sales & Marketing Director
FEATURED INSIGHTS
-
Behind every life-saving cell therapy is a complex manufacturing journey. Examine the critical role of automation in overcoming production challenges and ensuring quality.
-
Developing effective dry powder inhalers requires expertise across multiple disciplines, from particle engineering and device technology to manufacturing science and regulatory knowledge.
-
See how new technology tackles multiple fill-finish challenges, integrating robotics and vision systems for adaptable, contamination-controlled processing of ready-to-use pharmaceutical containers.
-
Space constraints and sterile integrity drive the shift toward modular, robotic manufacturing. Discover how adopting compact, automated systems reduces energy overhead and contamination risks.
-
Discover how innovations in closed-loop control and mechanical design extend peristaltic dosing limits to 2000 cP, maintaining accuracy while preserving the advantages of sterile, disposable paths.
-
Discover how pharmaceutical extrusion, including aseptic methods, enables advanced drug delivery systems, detailing the critical process controls required for success with complex and novel drug products.
-
By rethinking cleanroom requirements and reducing resource-heavy sterilization steps, manufacturers can significantly lower their Environmental Cost of Ownership and futureproof their operations.
-
Discover how a novel compression technique eliminates vacuum-related contamination and sealing failures in DPI blister manufacturing, leading to a cleaner, more accurate filling process.
-
Discover how filling spray-dried powders directly into syringes improves stability, minimizes cold chain reliance, and accelerates administration speeds for critical therapies.
-
Manual processes are yielding to automated, GMP-compliant closed systems in CGT manufacturing. This shift is crucial for meeting Annex 1 contamination standards and creating scalable, reliable commercial production.
-
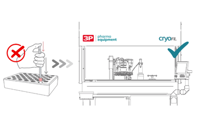
New sterile manufacturing rules require major changes to cryovial filling. Ensure compliance by adopting better "first-air" protection, minimizing human contact, and using 100% dose checks.
-
Discover how advanced API-in-capsule technology addresses previous limitations, providing a flexible and efficient path for drug candidates from development to clinical trials.
-
Manual cryovial filling in cell therapy is risky, time-consuming, and unsustainable. Learn why automating this crucial step is essential for precision, viability, and scalability.
-
Explore essential considerations for choosing the right sealing method in product development with insights on the benefits and drawbacks of different techniques for various industries.
-
Facing strict regulations and tricky powders, a healthcare client sought an advanced filling solution. Learn how an expert collaboration led to licensed product, boosting precision and productivity.
-
Discover the critical process considerations and custom engineering required to handle temperature-sensitive and high-viscosity pharmaceutical materials.
-
Beyond a simple mechanical step, crimping is a critical control point. We examine how advanced rotary technology provides superior seal quality, particulate control, and data-driven compliance assurance.
-
Patent expiry of original product enables 3P innovation to design enhanced equivalent DPI filling system for developers and CMOs.
-
Don't ignore Design for Manufacture (DfM) early on. Understand how initial design choices impact long-term costs, especially with regulatory hurdles and clinical trials locking in processes.
-
Discover how AI, Industry 4.0, and innovative drug containers are transforming the future of Advanced Therapy Medicinal Product (ATMP) manufacturing for better patient outcomes.
-
Delves into the evolution of Advanced Therapy Medicinal Products (ATMPs), exploring their complex development, revolutionary potential in treating diseases, and the intricacies of cell and gene therapies.
-
From Bunsen burners to advanced isolators, aseptic processing has evolved significantly. Delve into the evolution of this crucial manufacturing method, from its early sterilization techniques to today's highly controlled and regulated environments.
-
Investing in adaptable aseptic manufacturing pays off. Find out how flexible systems, despite higher initial costs, handle complex drugs and changing volumes to boost productivity and quality.
-
Designing pharmaceutical isolators requires navigating complex engineering hurdles, from ensuring chemical compatibility to optimizing airflow and pressure. Explore the critical considerations that guarantee drug manufacturing safety and efficiency.
-
Regulatory demands and patient well-being drive the necessity of aseptic techniques. Learn how these procedures, vital to pharmaceutical manufacturing, prevent contamination and ensure drug safety.
-
Producing viable cell-based therapies is a delicate science. Explore the fundamental culture processes, from initial cell extraction to final patient administration.
-
From personalized treatments to large-scale viral vector production, the future of ATMPs hinges on optimized automation. We delve into the technologies driving this transformation and the challenges ahead.
-
Pharmaceutical production of sterile powders presents unique hurdles. Explore the core principles and advanced techniques vital for aseptic filling, from containment to precise dosage control.