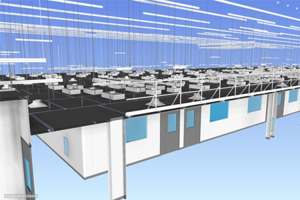
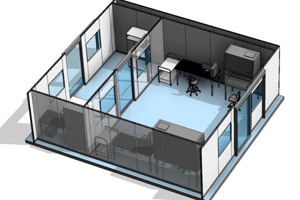
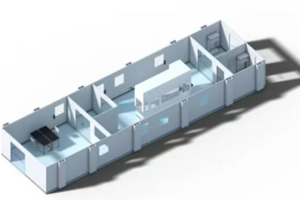
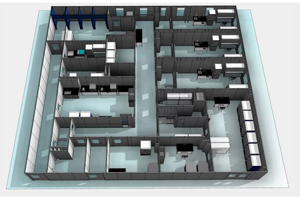
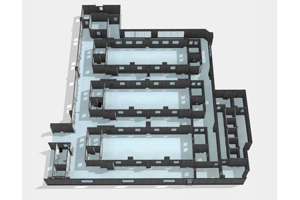
We are the only USA based cleanroom company that self-performs design, manufacture, and installation of cGMP modular cleanroom facilities. Working with AES, you can rest assured that your project is built on time and on budget, guaranteed. Our expertise in cleanroom solutions combined with our focus on your project success is second to none.
CONTACT INFORMATION
AES Cleanroom Technology
422 Stump Road
Montgomeryville, PA 18936
UNITED STATES
Phone: (215) 393-6810
Contact: Sales / Marketing
FEATURED ARTICLES
-
IoT-enabled smart BMS modernize biopharmaceutical cleanrooms through continuous monitoring, predictive analytics, energy efficiency, and strengthened regulatory compliance, enabling safer, more efficient operations.
-
Learn critical lessons from FDA 483 observations and Warning Letters to proactively address common GMP cleanroom compliance failures and build an inspection-ready facility.
-
AES Cleanroom Technology, an award-winning leader in modular cleanroom design and construction, has opened a new regional office in Research Triangle Park (RTP), reinforcing its commitment to serving the Southeast’s fast-growing life sciences, biotechnology, and advanced manufacturing sectors.
-
Learn essential conceptual design principles for transforming alternative spaces into efficient, regulatory-aligned cleanroom environments for your critical processes.
-
In this segment from the Pharmaceutical Online Live event, “Facility Design And Validation Considerations For Drug Manufacturers,” our panelists discuss where utilities fit into the construction and planning of a manufacturing facility. As panelist Fred Grossfeld explains, planning ahead can save you a lot of grief (and costs) in the long run.
-
In this segment from the Pharmaceutical Online Live event, “Facility Design And Validation Considerations For Drug Manufacturers,” panelist Herman Bozenhardt emphasizes the importance of HVAC systems for keeping workers, products, and the environment safe. He touches on the type of systems required for different biosafety levels and how to navigate the complex regulatory requirements across countries and municipalities.
-
In this segment from the Pharmaceutical Online Live event, “Facility Design And Validation Considerations For Drug Manufacturers,” our panelists respond to an audience question about designing a facility with future needs in mind.
-
Modular facility design offers a streamlined approach to construction, reducing the building time and headaches associated with stick-built facilities. These benefits come at a premium cost and more up-front planning, but if erecting a facility in record time is your goal, it may be worth it. In this segment from the Pharmaceutical Online Live event, “Facility Design And Validation Considerations For Drug Manufacturers,” our panelists weigh the benefits of the modular construction approach and share stories from first-hand experience.
-
In this segment from the Pharmaceutical Online Live event, “Facility Design And Validation Considerations For Drug Manufacturers,” our panelists discuss key considerations and common oversights in designing facilities for single-use systems. Things like differences in bioreactor size, tubing, drainage, waste flows, and maintenance all need to be factored into front-end planning.
-
AES Clean Technology, a leading provider of high-performance cleanroom facilities, has today announced the appointment of Jeff Rozelle as Senior Vice President of Business Development, West Region.
-
Gain insights into the nuances of cleanroom conceptual design for GMP operations and learn how an integrated approach can ensure regulatory compliance, functionality, and environmental performance.
-
Pharma Manufacturing announced the 2024 Pharma Innovation Award winners. This year’s winners consist of 13 products and technologies chosen by editors and industry reviewers.
-
Learn how you can obtain comprehensive solutions for cleanroom projects that help you ensure regulatory compliance and operational efficiency.
-
A leading provider of high-performance cleanroom facilities, AES Clean Technology, has appointed Chris Miller as Chief Executive Officer.
-
AES Clean Technology, a leading provider of high-performance cleanroom facilities, has launched its CleanLock Module™ today. This revolutionary airlock solution enhances cleanliness, speed, and efficiency in cleanroom project execution.
-
AES Clean Technology, a leading provider of high-performance modular cleanroom facilities, has appointed Chris Barbieri as Vice President of Engineering.
-
A leading provider of high-performance cleanroom facilities, AES Clean Technology, has announced the appointment of John Costalas to its senior leadership team as Vice President of Construction.
-
A leading provider of high-performance cleanroom facilities, AES Clean Technology, has appointed Brian Weed as Chief Revenue Officer (CRO).
-
Building the right cleanroom involves critical early decisions. Learn about a methodical framework ensuring flexibility, compliance, and operational readiness from initial concept through delivery.
-
Provider of high-performance cleanroom facilities for the pharmaceutical industry, AES Clean Technology, is celebrating two of its customers' success at this year's IPSE Facility of the Year Awards (FOYA), having designed and built the cleanrooms in both Genentech and Nexus Pharmaceuticals’ award-winning facilities.