ABOUT US



TSI serves the global pharmaceutical manufacturing market by providing trusted measurements in world class cleanrooms. Whether you periodically classify your clean areas or monitor them per your contamination control strategy, TSI particle counting instruments are here to help.
Now available, the Aerotrak™+ Portable Particle Counter A100 Series is specifically engineered for you to make your job easy, providing what you've been asking for in a portable particle counter, including:
- Easy compliance — EU GMP Annex 1 (2022 and 2008), ISO 14644-1:2015, and China GMP
- Data integrity — based on ALCOA+ principles
- Intuitive GUI — requires no manuals to operate, significantly reducing the chance for user error and simplifying the sampling process
- Communication options
The TSI BioTrak Real-Time Viable Particle Counter, a bio-fluorescent particle counter (BFPC), is the industry referenced instrument for real-time viable air and total particulate testing of pharmaceutical manufacturing environments. This instrument is your solution for meeting Annex 1 requirements for continuous viable air testing in Grade A areas to increase process understanding and reduce risk.
Industry trusted TSI FMS Software incorporates TSI particle counters, environmental sensors, real-time alarms and automated data collection. All work seamlessly as a complete system to assure easy FDA 21 CFR part 11 compliance and the highest level of data integrity. The continuous monitoring capabilities of aseptic processes reduces your risk by eliminating environmental monitoring (EM) interventions and improving quality through better process understanding.
With headquarters based in the U.S. and field offices throughout Europe and Asia, TSI has established a leading worldwide presence in the markets we serve.
CONTACT INFORMATION
TSI Incorporated
500 Cardigan Road
Shoreview, MN 55126
UNITED STATES
Phone: 800-777-8356
Contact: Sales
FEATURED ARTICLES
-
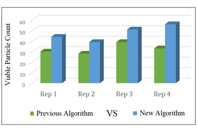
A new algorithm developed by a real-time viable particle counter determines the viability of a particle based on its measured optical properties, improving the detection of microorganisms.
-
To meet immediate vaccine demand, aseptic manufacturing capacity must increase quickly. This means environmental monitoring programs must adapt rapidly as well. Thoughtful planning can help.
-
Investigations into viable air excursions are difficult to perform using traditional methods. Discover why a real-time viable particle counter is key to providing process improvement.
-
GMP compliance requires monitoring microbial contamination levels in cleanrooms and clean spaces. It is important to know the role active air samplers play in this process and parameters for evaluating them.
-
Facility monitoring software with OPC UA client/server functionality makes great business sense. Learn how monitoring leads to reduced waste, improved yield, higher quality, and increased profits.
-
Real-time viable particle monitoring instruments have been available for over a decade. Learn in detail about this technology and how it is used to detect viable particles in real time.
-
Airborne microbial contamination is a serious concern for companies producing medicines and biotech products, and it must be addressed to ensure that products and people are kept safe.
-
A facility monitoring system is a process monitoring tool that collects data from sensors such as optical particle counters, differential pressure sensors, and temperature probes in realtime.
-
The BIOTRAK Particle Counter is a full-featured instrument that detects the total number of particles in the air as well as determines which of those particles are viable in nature.
-
Rapid microbiological methods have been around for years but have yet to make a significant impact on pharma manufacturing despite the advantages they offer in cost savings and process improvement.
-
Change is around the corner for pharmaceutical cleanrooms, as significant revisions to current ISO particle counting standards are forthcoming. With this in mind, Pharmaceutical Online thought it was a good time to speak with Troy Tillman of TSI, a supplier of particle counters and other precision measurement solutions.
-
At Interphex 2014, Todd and Todd interview Tim Russell, Field Market Developer with TSI Incorporated to discuss how to detect potential contamination events before they occur.
-
When specifying particle counters for continuous monitoring applications, there are many system design decisions to be made. Read about the advantages of using a remote particle counter.
-
The laser induced fluorescence signatures generated from environmental particles are complex. It is a challenging task to discriminate viable from non-viable particles that have fluorescence characteristics.
VIDEOS
-
Monitoring critical events and maintaining process compliance doesn't have to be a challenge. You can gain real-time visibility and control over your operations with a robust alarm management system.
-
Discover how you can visualize your cleanroom or factory, place sample points, and see live data in a dynamic, interactive floor plan.
-
Quickly visualize and access manufacturing data over time with customizable graphs. Prioritize critical information for faster, smarter quality decisions.
-
Understand your environment quickly with real-time, customizable data and interactive controls, whether you manage a single site or multiple locations.
-
Remotely monitor cleanroom environments with secure, real-time data access from any device, anywhere, helping to streamline operations and ensure compliance.
-
Discover a portable particle counter that enables you to create a scheduled workflow and ensure you never miss a sample during cleanroom monitoring or sample the wrong area.
-
Watch to see how easy it is to start collecting data according to your standard operating procedure with a portable particle counter.
-
Discover a particle counter that automatically determines the number of sample locations needed based on the sampling size of the area, enabling the easy selection of classification for certification.
-
Learn how you can easily create and download a report with a portable particle monitor for easy compliance in your cleanroom.
-
Discover a portable particle counter with a sample tracking feature that enables you to easily view and receive real-time alerts during the process when certifying or monitoring your cleanroom.
-
With the press of a button, you can immediately start taking particle counts in your cleanroom using a portable particle counter for easy cleanroom monitoring.
-
Discover a portable particle counter for quick and easy cleanroom monitoring that features automated alarms and reporting to help you ensure compliance.
-
Classification plays a vital role in cleanroom certification and qualification. Discover a particle counter that simplifies testing for adherence to ISO 14644-1, EU GMP Annex 1, or China GMP standards.
-
Learn how cleanroom technicians can perform confident, reliable aseptic microbial monitoring in pharmaceutical manufacturing Grade A and B environments with external vacuum systems.
-
Here, we introduce a real-time viable particle monitor and how it can quickly identify the source of contamination in pharmaceutical cleanrooms.
-
Watch to learn about the BIOTRAK® Real-Time Viable Pharmaceutical Particle Counter from TSI, which provides real-time information on microbiological contamination in pharmaceutical cleanrooms.
-
Discover a portable particle counter that ensures your classification testing complies with the revised EU GMP Annex 1 with updated limits and the ability to define limits that are no longer specified.