Pharmaceutical Package Inspection
FEATURED ARTICLES
-
Engineering Excellence In Container Closure Integrity Testing
Explore the critical factors that define a reliable container closure integrity method, as well as what truly distinguishes robust, reproducible testing in today’s demanding parenteral landscape.
WHITE PAPERS & CASE STUDIES
-
Container Closure Integrity Testing: Sensitivity, Automation, Efficiency
Explore the need for sensitive, reliable, and automated container closure integrity testing technologies, and how existing deterministic solutions can help achieve optimum quality assurance goals.
- Leak Testing Of Pharmaceutical Packaging: Laser Based Technology vs. Conventional Blue Dye
- Leading Pharmaceuticals Manufacturer Eases The Time Burden Of Proofreading With New Software
- CCIT Comparative Study Between Dye Ingress And Deterministic Methods
- Detecting Integrity Breaches In A Range Of Pharmaceutical Blister Package Types
PRODUCTS AND SERVICES
-
Used Fortress Technology Phantom Metal Check Detector on Mobile Stand. Asset# EN 200240.
-
V57- Customizable, precise quality control
Immediate contamination detection and precision formation inspections of rigid plastic containers.
Advanced Product Handling
Product tracking, product handling options, and intuitive software enable efficient visual inspection of every product.
Real Time Quality Control
Installed directly after product formation, the system minimizes chances that misformed or contaminated products continue to downstream processes.
Precise Package Presentation
Detect product contamination as small as 0.1 TAPPI for top-quality products.
-
For the efficient and reproducible testing of your barrier system.
-
Anritsu's aerosol checkweigher is engineered for accurate weighing of aerosol cans in pharmaceutical applications. It features a star wheel mechanism that feeds cans onto the weigh table at a constant speed and with uniform spacing. This design, combined with a high precision electromagnetic weigh cell, ensures a throughput of up to 150 cans per minute, with a weighing accuracy of ± 10 mg. The checkweigher has a compact footprint, integrating within the same frame a reject mechanism and confirmation function, ensuring that only correctly weighed products pass through. Underweight and overweight cans are automatically directed into two separate bins located beneath the weigh table. The system is compliant with federal regulation 21 CFR Part 11 for the integrity of electronic records and signatures, with features such as password authentication, audit trail of operation data, and encryption/decryption of exported data.
-
The Food and Drug Administration (FDA), United States Pharmacopeia (USP) and EMA Annex 1 issue strict guidelines for testing the integrity of IV bags and containers closed by fusion. IV bag manufacturers are one among many sterile product manufacturers that must meet these test requirements for container closure integrity.
-
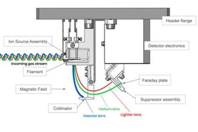
Helium leak detection (HeLD) is routinely used and widely accepted for applications that require the utmost leak sensitivity.
-
Identify defects and reduce false-rejects of bottled products with 360° vision inspection.
-
Parenteral products, specifically vials, both glass and, increasingly, polymeric vials, represent one of the most common package systems historically tested by helium leak detection, largely due to their continued dominance as a package system for high-potency drugs requiring utmost protection. The primary sealing interface of a traditional vial system is between the elastomeric closure and land seal of a vial, physically compressed together by a crimped aluminum seal.