ABOUT THERMO FISHER SCIENTIFIC
Thermo Fisher is a leading global provider of bioprocessing solutions. We purposefully connect our capabilities across all phases of the drug lifecycle, accelerating our customers’ pace to patient impact. With our comprehensive portfolio of products and services we are a dedicated partner throughout the biological drug development journey.
Key Capabilities:
- Media and buffer preparation
- Cell expansion
- Cell culture production
- Harvest
- Capture and polish chromatography
- Viral inactivation, filtration, and QC testing
- Bulk storage, fill, and finish
- cGMP chemicals and supply chain services
- Expert professional support to meet process, scale, quality, and regulatory requirements
CONTACT INFORMATION
Thermo Fisher Scientific
7305 Executive Way
Frederick, MD 21704
UNITED STATES
Contact: Hunter Tuck
FEATURED SOLUTIONS
-
Leverage diverse, ready-to-use media and feed formulations to quickly identify the optimal nutrient balance for your CHO cell line, accelerating bioproduction success and subsequent process optimization.
-
Leverage custom media development services to optimize formulations for increased titers, quality, and manufacturability. Choose between traditional or multi-omics workflows to meet your cell line's specific needs.
-
Maintain material quality and integrity with storage facilities compliant with ISO 9001, cGMP, and GDP standards. Features include real-time temperature control and HAZ-class segregation.
-
Effective inventory management ensures the quality and traceability of raw materials, supports regulatory compliance, and enables operational savings. Learn how to secure your supply chain and reduce stockouts.
-
Transitioning to biobased materials in cell culture reduces reliance on virgin fossil fuel feedstocks and helps meet Scope 3 emission reduction goals, offering a sustainable path for biomanufacturing.
-
When working in a critical environment such as a clean room or when producing critical bioproducts, even microscopic amounts of contamination can have a disastrous impact. The tiered portfolio of Thermo Scientific™ Nalgene™ containers provides bioproduction facilities with a range of high-quality sterile storage and transport options while addressing particulate level (USP <788>), endotoxin level, and sterility requirements
-
This single-use bioreactor increases efficiency in process development by eliminating cleaning and autoclaving steps. Learn how it can help maximize cell density for fed-batch and perfusion processes.
-
Our comprehensive network of manufacturing facilities enables us to offer robust supply assurance and consistent product quality to biopharmaceutical developers around the globe.
FEATURED INSIGHTS
-
Amid the mRNA and oligonucleotide therapeutic boom, sponsors and manufacturers are exploring how to make critical raw materials—capping agents, lipid nanoparticles, and RNA polymerase—more effective.
-
Residual host-cell DNA quantitation requires sensitive, well-validated analytical workflows to ensure accurate impurity control, process understanding, and regulatory compliance in complex biologics.
-
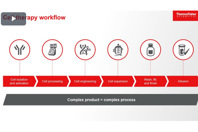
The most common contamination risks in cell therapy manufacturing are open processes that leverage different products, inflexible instruments, labor intensive workflows, lack of in-line monitoring, and zero failure tolerance.
-
Process robust mAb production requires understanding scale dependent cell environments, leveraging predictive models, and pursuing data driven control to minimize variability, optimize quality, and ensure reliable technology transfer across biomanufacturing scales.
-
Multispecific antibodies are a newer class of cancer therapeutics addressing tumor complexity. Learn about the design, manufacturing, and regulatory challenges and integrated solutions accelerating their development.
-
Industry 5.0 represents the next generation of bioproduction—the Adaptive Plant—where human expertise is empowered by AI-enabled insights, autonomous systems, and strategic collaboration.
-
Outsourcing your cGMP chemical supply chain can significantly reduce capital and operating expenses, minimize production delays, and boost manufacturing productivity through improved sourcing and logistics.
-
Due to the complexity of bispecifics, Fc-fusion proteins, and Fab fragments, their manufacturing poses added purification challenges and requires a robust toolkit of purification techniques.