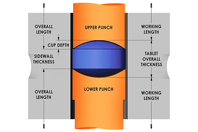
ABOUT US
Natoli Engineering Company, Inc., is the recognized global leader in tablet press tooling manufacturing. Founded on the uncompromising principle to manufacture and deliver only the highest quality tablet compression tooling, presses and replacement parts at a fair price with exceptional customer service, Natoli has been at the forefront of the tablet compression industry for over 40 years. Our state-of-the-art manufacturing facilities, award-winning engineering team and dedicated production crew enables us to offer clients competitive pricing without compromising our high standards of customer service. Trust in Natoli – our staff has the industry knowledge and expertise to understand and meet your exact requirements and requests.
PRODUCT INFORMATION
- Robust Mid-Sized Tablet Press
- Encapsulator For Separation, Filling, And Polishing
- Pharmaceutical Double-Sided Rotary Tablet Press
- Single-Station Hydraulic Pharmaceutical Tablet Press: NP-P20A Brochure
- Pharmaceutical Encapsulation Change Parts And Kits Catalog
- Tablet Compression Accessories Catalog 7th Edition
- Natoli AIM™ Data Acquisition & Analytical Software Datasheet
- Pharmaceutical Metallurgical Services Brochure
- Natoli Scientific Brochure
- Natoli NP-500 Rotary Tablet Press Brochure
- Data Acquisition And Analytical And Operating System Software
- Tablet Press For Laboratory Research & Development
- Pharmaceutical Tablet Press: NP-155 Brochure
- Natoli Tablet Press Replacement Parts Brochure
- Digitial Inspection Microscope Brochure
- Tablet Press Refurbishing Services
- Laser Vision System (LVS) Punch Inspection Device
- Rotary Tablet Press: NP-400
CONTACT INFORMATION
Natoli Engineering Company, Inc.
28 Research Park Circle
St. Charles, MO 63304
UNITED STATES
Phone: 636-926-8900
Fax: 636-926-8910
Contact: Dale Natoli
ARTICLES
-
Many growing industries face universal tablet compression challenges, demanding better tooling, tighter process control, and more integrated data to ensure product consistency and reliable scale-up.
-
New materials, smart automation, and in-press quality control are optimizing tablet compression. These five key innovations are driving efficiency and consistency in solid dose manufacturing today.
-
Unlock manufacturing precision with digital twins. Learn how virtual replicas of production systems enable predictive maintenance, streamline scale-up, and ensure consistent product quality in pharma.
-
Discover how continuous manufacturing, AI-driven digital twins, and advanced tooling materials are transforming tablet compression to boost quality and cut operational costs in pharmaceutical production.
-
Non-uniform density causes many tablet failures. Learn how optimizing your formulation, tablet proportions, and tooling design ensures proper de-aeration for consistent quality.
-
Segments increase press output by up to 25% and cut setup time by 88%. Learn the benefits, drawbacks, and manufacturing needs of this rapidly growing tablet press technology.
-
Tablet consistency relies on a balance of formulation, press mechanics, and tooling. Learn why punch length tolerance is often overstated and what other critical factors truly drive quality and uniformity.
-
When manufacturing mini-tablets, adjusting punch force ratings is crucial to prevent bending or buckling. The Rankine-Gordon formula offers a safer force rating for microtip tooling.
-
The pressure-sensitive bisect design allows accurate tablet splitting by maximizing facet width, optimizing angles, and balancing radius values for durability and user-friendly administration.
-
Addressing tablet edge erosion and friability involves adjusting formulation, tablet design, tooling, press setup, and operation speed to improve tablet robustness and reduce weight loss.
-
Tablet debossing aids medication identification and must meet FDA standards. Font, scale, color, shadow, and stroke details are key for optimal legibility and safety.
-
Accurately setting the lower punch ejection height in your tablet press is crucial to the ejection process. Explore how to avoid poorly positioning your punch.
-
Picking and sticking are the most common tablet defects that can reduce your product quality and performance. This makes perfecting the right pre-picking strategy essential to improving your processes.
-
What makes the keying in tablet compression tooling, specifically in tablet punches, so important to the tablet manufacturing processes?
-
What is head pressure in your hopper and how does it relate to tablet press performance?
-
Die bore cracking is a significant issue in tablet manufacturing, affecting the quality of the tablets and potentially damaging the tablet press. Explore how to analyze for and prevent this damage.
-
Dust cups and bellows are essential components in tablet manufacturing, utilized to prevent lubricants from contaminating the tablets.
-
Explore maximum compression force as it relates to the type of tool steel being used and the properties of the tablet punch materials.
-
Discover best practices for overcoming sticky formulation issues in capsule filling. This guide offers step-by-step troubleshooting methods to ensure smooth and efficient encapsulation processes.
-
Explore how understanding specific USP chapters can address common tablet and capsule production challenges and ensure high-quality results in this essential pharmaceutical process.
-
Knowing how formulation moves through a tablet process is invaluable in optimizing the process for the greatest tablet quality and production.
-
"Tablet sticking" presents an ongoing challenge for tablet manufacturers. While the science behind this occurrence is complex, understanding it is an essential part of ensuring high-quality tablet production.
-
Quality by Design (QbD) ensures efficiency and high standards in pharmaceutical tablet development. Explore how this proactive approach transforms manufacturing processes and enhances product quality.
-
Ensure your press is running with optimal efficiency by expanding your knowledge of proper cleaning and maintenance.
-
Scaling a new drug formulation from development to manufacturing often presents challenges, but there are ways to minimize deviations and streamline your transition.
WHITE PAPERS
-
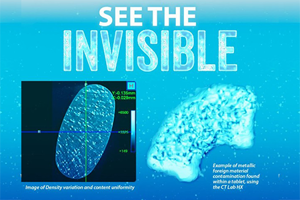
Discover how X-ray micro-CT provides vital, non-destructive analysis of a tablet’s internal 3D physical structure. This structural insight is essential for troubleshooting defects and confirming performance.
-
Review strategies to mitigate sticking and picking risks in the tablet industry, and learn how you can foster improved tablet production processes, ensuring higher quality and consistency.
-
Learn how a partner company that is committed to assisting manufacturers with calibration and optimal set-point establishment can serve as a valuable resource in achieving optimal tablet production.
-
Why is a horizontal optical comparator highly recommended for tablet manufacturers as a measuring tool for punch tip inspection, and what strategies can be used to detect, reduce, and prevent tip wear?
-
Explore the extended head flat and how this punch modification can improve the compression of difficult formulations.
-
Knowing how to use USP <1062> helps manufacturers manage the wide variety of factors that can exacerbate production costs, or result in no product at all.
VIDEOS
- Maximize Uptime And Yield With This Feeder Base Leveling System
- FastLok™ Die Table Surface Overview
- Boost Quality For More Efficient Tablet Manufacturing
- Tablet Characterization With Regulatory Compliance Method USP<1062>
- Overcoming Solid Dose Formulation And Tablet Compression Issues
- Tablet Tooling Video Series: How To Assemble And Disassemble A Multi-Tip Assembly Punch
- How-To Video: Shaped Tooling Installation & Tablet Press Setup
- How To Video: Install Round Tooling And Set Up A Tablet Press
- How-To Video: Multi-Tip Tooling Installation & Tablet Press Setup